Direct detection of protein biomarkers in human fluids using site-specific antibody immobilization strategies
- PMID: 24481229
- PMCID: PMC3958245
- DOI: 10.3390/s140202239
Direct detection of protein biomarkers in human fluids using site-specific antibody immobilization strategies
Abstract
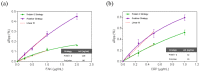
Design of an optimal surface biofunctionalization still remains an important challenge for the application of biosensors in clinical practice and therapeutic follow-up. Optical biosensors offer real-time monitoring and highly sensitive label-free analysis, along with great potential to be transferred to portable devices. When applied in direct immunoassays, their analytical features depend strongly on the antibody immobilization strategy. A strategy for correct immobilization of antibodies based on the use of ProLinker™ has been evaluated and optimized in terms of sensitivity, selectivity, stability and reproducibility. Special effort has been focused on avoiding antibody manipulation, preventing nonspecific adsorption and obtaining a robust biosurface with regeneration capabilities. ProLinker™-based approach has demonstrated to fulfill those crucial requirements and, in combination with PEG-derivative compounds, has shown encouraging results for direct detection in biological fluids, such as pure urine or diluted serum. Furthermore, we have implemented the ProLinker™ strategy to a novel nanoplasmonic-based biosensor resulting in promising advantages for its application in clinical and biomedical diagnosis.
Figures






References
-
- Estevez M.C., Alvarez M., Lechuga L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photon. Rev. 2012;6:463–487.
-
- Estevez M.C., Otte M.A., Sepulveda B., Lechuga L.M. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal. Chim. Acta. 2014;806:55–73. - PubMed
-
- Wong L.S., Khan F., Micklefield J. Selective covalent protein immobilization: Strategies and applications. Chem. Rev. 2009;109:4025–4053. - PubMed
-
- Jung Y., Jeong J.Y., Chung B.H. Recent advances in immobilization methods of antibodies on solid supports. Analyst. 2008;133:697–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

