Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses
- PMID: 24481253
- PMCID: PMC3932872
- DOI: 10.1073/pnas.1324140111
Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses
Abstract
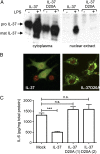
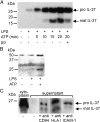
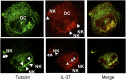
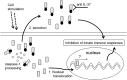
IL-37 is a fundamental inhibitor of innate immunity. Human IL-37 has a caspase-1 cleavage site and translocates to the nucleus upon LPS stimulation. Here, we investigated whether caspase-1 processing affects IL-37-mediated suppression of LPS-induced cytokines and the release from cells by analyzing a caspase-1 cleavage site mutant IL-37 (IL-37D20A). Nuclear translocation of IL-37D20A is significantly impaired compared with WT IL-37 in transfected cells. LPS-induced IL-6 was decreased in cells expressing WT IL-37 but not IL-37D20A. The function of IL-37 in transfected bone marrow-derived macrophages is nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome-dependent, because IL-37 transfection in apoptosis-associated speck-like protein containing a carboxyl-terminal caspase recruitment domain- and NLRP3-deficient cells does not reduce levels of IL-6 and IL-1β upon LPS stimulation. IL-37-expressing macrophages release both precursor and mature IL-37, but only the externalization of mature IL-37 was dependent on ATP. Precursor and mature IL-37 was also secreted from human dendritic cells and peripheral blood mononuclear cells. To determine whether IL-37 is active in the extracellular compartment, we pretreated IL-37 transgenic mice with IL-37-neutralizing antibodies before LPS challenge. In IL-37-expressing mice, neutralizing IL-37 antibodies reversed the suppression of LPS-induced serum IL-6. In contrast, the addition of neutralizing antibody did not reverse suppression of LPS-induced IL-6 in mouse macrophages transfected with IL-37. Although caspase-1 is required for nuclear translocation of intracellular IL-37 and for secretion of mature IL-37, the release of the IL-37 precursor is independent of caspase-1 activation. IL-37 now emerges as a dual-function cytokine with intra- and extracellular properties for suppressing innate inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boraschi D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22(3):127–147. - PubMed
-
- Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. - PubMed
-
- Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–327. - PubMed
-
- Kumar S, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18(2):61–71. - PubMed
-
- Sharma S, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180(8):5477–5482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

