Direct effect of sodium iodate on neurosensory retina
- PMID: 24481259
- PMCID: PMC4049579
- DOI: 10.1167/iovs.13-13075
Direct effect of sodium iodate on neurosensory retina
Abstract
Purpose: To systematically characterize the effects of NaIO3 on retinal morphology and function.
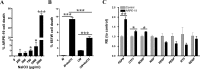
Methods: NaIO3 at 10, 20, or 30 mg/kg was administered by retro-orbital injection into adult C57BL/6J mice. Phenotypic and functional changes of the retina were assessed at 1, 3, 5, and 8 days postinjection by fundus imaging, optical coherence tomography (OCT), ERG, and histology. Direct NaIO3 cytotoxicity on ARPE-19 and 661W cells was quantified using lactate dehydrogenase (LDH) apoptosis assay. Effect of NaIO3 on RPE and photoreceptor gene expression was assessed in vitro and in vivo by quantitative PCR.
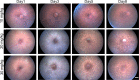
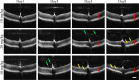
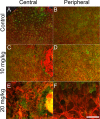
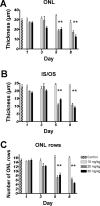
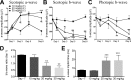
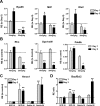
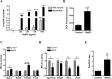
Results: While little to no change was observed in the 10 mg/kg NaIO3-injected group, significant retinal anomalies, such as RPE atrophy and retinal thinning, were observed in both 20 and 30 mg/kg NaIO3-injected groups. Gene expression analysis showed rapid downregulation of RPE-specific genes, increase in heme oxygenase 1 expression, and induction of the ratio of Bax to Bcl-2. Electroretinographic response loss and photoreceptor gene repression preceded gross morphological changes. High NaIO3 toxicity on 661W cells was observed in vitro along with reactive oxygen species (ROS) induction. NaIO3 treatment also disrupted oxidative stress, phototransduction, and apoptosis gene expression in 661W cells. Exposure of ARPE-19 cells to NaIO3 increased expression of neurotrophins and protected photoreceptors from direct NaIO3 cytotoxicity.
Conclusions: Systematic characterization of changes associated with NaIO3 injection revealed a large variability in the severity of toxicity induced. Treatment with >20 mg/kg NaIO3 induced visual dysfunction associated with rapid suppression of phototransduction genes and induced oxidative stress in photoreceptors. These results suggest that NaIO3 can directly alter photoreceptor function and survival.
Keywords: cytotoxicity; oxidative stress; photoreceptor; retinal degeneration; sodium iodate.
Figures

References
-
- Samardzija M, Neuhauss SCF, Joly S, Kurz-Levin M, Grimm C. Animal models for retinal degeneration. In: Animal Models for Retinal Diseases. Totowa, NJ: Humana Press; 2009. Neuromethods; vol 46: 51–79
-
- Zeiss CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet Pathol. 2010; 47: 396–413 - PubMed
-
- Webster SH, Rice ME, Highman B, Von Oettingen WF. The toxicology of potassium and sodium iodates: acute toxicity in mice. J Pharmacol Exp Ther. 1957; 120: 171–178 - PubMed
-
- Enzmann V, Row BW, Yamauchi Y, et al. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006; 82: 441–448 - PubMed
-
- Mizota A, Adachi-Usami E. Functional recovery of retina after sodium iodate injection in mice. Vision Res. 1997; 37: 1859–1865 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

