N-WASP-directed actin polymerization activates Cas phosphorylation and lamellipodium spreading
- PMID: 24481817
- PMCID: PMC3970555
- DOI: 10.1242/jcs.134692
N-WASP-directed actin polymerization activates Cas phosphorylation and lamellipodium spreading
Abstract
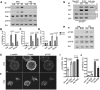
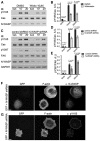
Tyrosine phosphorylation of the substrate domain of Cas (CasSD) correlates with increased cell migration in healthy and diseased cells. Here, we address the mechanism leading to the phosphorylation of CasSD in the context of fibronectin-induced early spreading of fibroblasts. We have previously demonstrated that mechanical stretching of CasSD exposes phosphorylation sites for Src family kinases (SFKs). Surprisingly, phosphorylation of CasSD was independent of myosin contractile activity but dependent on actin polymerization. Furthermore, we found that CasSD phosphorylation in the early stages of cell spreading required: (1) integrin anchorage and integrin-mediated activation of SFKs, (2) association of Cas with focal adhesion kinase (FAK), and (3) N-WASP-driven actin-assembly activity. These findings, and analyses of the interactions of the Cas domains, indicate that the N-terminus of Cas associates with the FAK-N-WASP complex at the protrusive edge of the cell and that the C-terminus of Cas associates with the immobilized integrin-SFK cluster. Thus, extension of the leading edge mediated by actin polymerization could stretch Cas during early cell spreading, priming it for phosphorylation.
Keywords: Actin dynamics; BCAR1; Cas; Crk-associatied substrate; FAK; Focal adhesion kinase; SFK; Src family kinase; p130Cas.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

