Regulation of Src trafficking and activation by the endocytic regulatory proteins MICAL-L1 and EHD1
- PMID: 24481818
- PMCID: PMC3986674
- DOI: 10.1242/jcs.133892
Regulation of Src trafficking and activation by the endocytic regulatory proteins MICAL-L1 and EHD1
Abstract
Localization of the non-receptor tyrosine kinase Src to the cell periphery is required for its activation and to mediate focal adhesion turnover, cell spreading and migration. Inactive Src localizes to a perinuclear compartment and the movement of Src to the plasma membrane is mediated by endocytic transport. However, the precise pathways and regulatory proteins that are responsible for SRC transport are incompletely understood. Here, we demonstrate that Src partially colocalizes with the endocytic regulatory protein MICAL-L1 (molecule interacting with CasL-like protein 1) in mammalian cells. Furthermore, MICAL-L1 is required for growth-factor- and integrin-induced Src activation and transport to the cell periphery in HeLa cells and human fibroblasts. Accordingly, MICAL-L1 depletion impairs focal adhesion turnover, cell spreading and cell migration. Interestingly, we find that the MICAL-L1 interaction partner EHD1 (EH domain-containing protein 1) is also required for Src activation and transport. Moreover, the MICAL-L1-mediated recruitment of EHD1 to Src-containing recycling endosomes is required for the release of Src from the perinuclear endocytic recycling compartment in response to growth factor stimulation. Our study sheds new light on the mechanism by which Src is transported to the plasma membrane and activated, and provides a new function for MICAL-L1 and EHD1 in the regulation of intracellular non-receptor tyrosine kinases.
Keywords: Circular dorsal ruffle; EHD1; Endocytic recycling; Focal adhesion; MICAL-L1; Migration; Src.
Figures

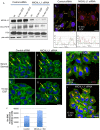



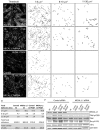

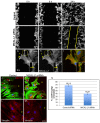

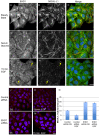

Similar articles
-
Endocytosis and the Src family of non-receptor tyrosine kinases.Biomol Concepts. 2014 May;5(2):143-55. doi: 10.1515/bmc-2014-0003. Biomol Concepts. 2014. PMID: 25372749 Free PMC article. Review.
-
Novel functions for the endocytic regulatory proteins MICAL-L1 and EHD1 in mitosis.Traffic. 2015 Jan;16(1):48-67. doi: 10.1111/tra.12234. Epub 2014 Nov 14. Traffic. 2015. PMID: 25287187 Free PMC article.
-
MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling.Mol Biol Cell. 2009 Dec;20(24):5181-94. doi: 10.1091/mbc.e09-06-0535. Mol Biol Cell. 2009. PMID: 19864458 Free PMC article.
-
Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors.J Biol Chem. 2010 Oct 15;285(42):31918-22. doi: 10.1074/jbc.C110.166066. Epub 2010 Aug 27. J Biol Chem. 2010. PMID: 20801876 Free PMC article.
-
Endocytic membrane trafficking in the control of centrosome function.Curr Opin Cell Biol. 2020 Aug;65:150-155. doi: 10.1016/j.ceb.2020.01.009. Epub 2020 Mar 3. Curr Opin Cell Biol. 2020. PMID: 32143977 Free PMC article. Review.
Cited by
-
The Seminiferous Epithelial Cycle of Spermatogenesis: Role of Non-receptor Tyrosine Kinases.Adv Exp Med Biol. 2021;1288:1-20. doi: 10.1007/978-3-030-77779-1_1. Adv Exp Med Biol. 2021. PMID: 34453729
-
Epigenetic induction of lipocalin 2 expression drives acquired resistance to 5-fluorouracil in colorectal cancer through integrin β3/SRC pathway.Oncogene. 2021 Nov;40(45):6369-6380. doi: 10.1038/s41388-021-02029-4. Epub 2021 Sep 29. Oncogene. 2021. PMID: 34588619
-
Human Papillomavirus 16 Infection Induces VAP-Dependent Endosomal Tubulation.J Virol. 2018 Feb 26;92(6):e01514-17. doi: 10.1128/JVI.01514-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29321327 Free PMC article.
-
Endocytosis and the Src family of non-receptor tyrosine kinases.Biomol Concepts. 2014 May;5(2):143-55. doi: 10.1515/bmc-2014-0003. Biomol Concepts. 2014. PMID: 25372749 Free PMC article. Review.
-
Spatial cycles mediated by UNC119 solubilisation maintain Src family kinases plasma membrane localisation.Nat Commun. 2017 Jul 24;8(1):114. doi: 10.1038/s41467-017-00116-3. Nat Commun. 2017. PMID: 28740133 Free PMC article.
References
-
- Cai B., Giridharan S. S., Zhang J., Saxena S., Bahl K., Schmidt J. A., Sorgen P. L., Guo W., Naslavsky N., Caplan S. (2013). Differential roles of C-terminal Eps15 homology domain proteins as vesiculators and tubulators of recycling endosomes. J. Biol. Chem. 288, 30172–30180 10.1074/jbc.M113.488627 - DOI - PMC - PubMed
-
- Caplan S., Naslavsky N., Hartnell L. M., Lodge R., Polishchuk R. S., Donaldson J. G., Bonifacino J. S. (2002). A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 21, 2557–2567 10.1093/emboj/21.11.2557 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

