Rationale and design of LAPLACE-2: a phase 3, randomized, double-blind, placebo- and ezetimibe-controlled trial evaluating the efficacy and safety of evolocumab in subjects with hypercholesterolemia on background statin therapy
- PMID: 24481874
- PMCID: PMC6649582
- DOI: 10.1002/clc.22252
Rationale and design of LAPLACE-2: a phase 3, randomized, double-blind, placebo- and ezetimibe-controlled trial evaluating the efficacy and safety of evolocumab in subjects with hypercholesterolemia on background statin therapy
Abstract
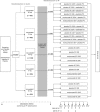
Low-density lipoprotein cholesterol (LDL-C) levels are significantly associated with atherosclerotic cardiovascular disease (ASCVD) risk, and studies using interventions that lower LDL-C levels have been shown to reduce the risk of ASCVD events and mortality. Statin treatment is the current first-line therapy for lowering LDL-C and reducing ASCVD risk. However, many patients are still unable to reach recommended LDL-C goals on maximally tolerated statin therapy. Monoclonal antibodies that inhibit proprotein convertase subtilisin/kexin type 9, including evolocumab (previously AMG 145), dramatically lowered LDL-C in phase 2 clinical trials when administered alone or in combination with a statin. The aim of this phase 3 study is to evaluate the efficacy of 12 weeks of subcutaneous evolocumab (vs placebo) administered every 2 weeks or every month in combination with a statin in patients with hypercholesterolemia and mixed dyslipidemia. This study will also provide comparative efficacy, safety, and tolerability data between evolocumab and ezetimibe when added to background atorvastatin therapy.
© 2014 Wiley Periodicals, Inc.
Figures
References
-
- Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. - PubMed
-
- Pekkanen J, Linn S, Heiss G, et al. Ten‐year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. - PubMed
-
- Grundy SM, Cleeman JI, Merz CN, et al; for the Coordinating Committee of the National Cholesterol Education Program, endorsed by the National Heart, Lung, and Blood Institute, American College of Cardiology Foundation, and American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110:763]. Circulation. 2004;110:227–239. - PubMed
-
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention and Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012) . The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) [published correction appears in Eur Heart J. 2012;33:2126]. Eur Heart J. 2012;33:1635–1701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

