Ovulation triggers in anovulatory women undergoing ovulation induction
- PMID: 24482059
- PMCID: PMC11056233
- DOI: 10.1002/14651858.CD006900.pub3
Ovulation triggers in anovulatory women undergoing ovulation induction
Abstract
Background: Anovulation is a common cause of infertility. Drugs used to treat anovulation include selective oestrogen receptor modulators, aromatase inhibitors and gonadotrophins. Ovulation triggers are used with these drugs, as a surrogate for the hormonal surge seen in spontaneous menstrual cycles, to control the timing of ovulation and the timing of sexual intercourse. Ovulation triggers given without reliable evidence of oocyte maturity could be inappropriately timed; they increase costs, and the need to time intercourse precisely after the ovulation trigger is given adds to psychological stress.This is an update of a Cochrane review first published in Issue 3, 2008, of the Cochrane Database of Systematic Reviews.
Objectives: To determine the benefits and harms of administering an ovulation trigger to anovulatory women receiving treatment with ovulation-inducing agents in comparison with spontaneous ovulation following ovulation induction.
Search methods: We updated searches of the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO to November 2013. We checked conference proceedings, trial registries and reference lists and contacted researchers.
Selection criteria: Parallel-group, randomised, controlled trials (RCTs) evaluating the administration of an ovulation trigger to anovulatory women receiving treatment with ovulation-inducing agents.
Data collection and analysis: We independently assessed trial eligibility and trial quality and extracted data. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous data and used the random-effects model in meta-analyses when significant heterogeneity was present. We assessed overall quality of the evidence by using the GRADE approach.
Main results: No new trials were identified. This review includes two RCTs with low risk of bias that compared urinary human chorionic gonadotrophin (hCG) versus no treatment in anovulatory women receiving clomiphene citrate. Urinary hCG did not result in an increase in live birth rate over no hCG (OR 0.97, 95% CI 0.52 to 1.83; two trials, 305 participants, I(2) = 16%; low-quality evidence), but very serious imprecision around the effect estimate reduces our confidence in the apparent lack of effect of hCG as an ovulation trigger in clomiphene-induced cycles in anovulatory women.Among this review's secondary outcomes, urinary hCG may not increase ovulation rate (OR 0.99, 95% CI 0.36 to 2.77; two trials, 305 participants, I(2) = 55%; low-quality evidence), clinical pregnancy rate (OR 1.02, 95% CI 0.56 to 1.89; two trials, 305 participants, I(2) = 35%; low-quality evidence) or miscarriage rate in pregnant women (OR 1.19, 95% CI 0.17 to 8.23; two trials, 54 participants, I(2) = 0%; low-quality evidence). Multiple pregnancies and preterm deliveries were uncommon, and ovarian hyperstimulation syndrome, adverse events and deaths were not reported as outcomes in either trial. We found no trials evaluating other ovulation triggers.
Authors' conclusions: Evidence is inadequate to recommend or refute the use of urinary hCG as an ovulation trigger in anovulatory women treated with clomiphene citrate. We found no trials evaluating the use of ovulation triggers in anovulatory women treated with other ovulation-inducing agents.
Conflict of interest statement
KG and RN are authors of a published randomised trial (George 2007) that is included in this review. The other review authors (MSK and PT) are not aware of any conflict of interest.
Figures



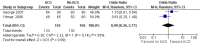








Update of
-
Ovulation triggers in anovulatory women undergoing ovulation induction.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD006900. doi: 10.1002/14651858.CD006900.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 Jan 31;(1):CD006900. doi: 10.1002/14651858.CD006900.pub3. PMID: 18646175 Updated.
Similar articles
-
Ovulation triggers in anovulatory women undergoing ovulation induction.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD006900. doi: 10.1002/14651858.CD006900.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 Jan 31;(1):CD006900. doi: 10.1002/14651858.CD006900.pub3. PMID: 18646175 Updated.
-
Aromatase inhibitors (letrozole) for ovulation induction in infertile women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2022 Sep 27;9(9):CD010287. doi: 10.1002/14651858.CD010287.pub4. Cochrane Database Syst Rev. 2022. PMID: 36165742 Free PMC article.
-
Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2018 May 24;5(5):CD010287. doi: 10.1002/14651858.CD010287.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Sep 27;9:CD010287. doi: 10.1002/14651858.CD010287.pub4. PMID: 29797697 Free PMC article. Updated.
-
Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility.Cochrane Database Syst Rev. 2017 Nov 29;11(11):CD003053. doi: 10.1002/14651858.CD003053.pub6. Cochrane Database Syst Rev. 2017. PMID: 29183107 Free PMC article.
-
Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome.Cochrane Database Syst Rev. 2016 Dec 15;12(12):CD002249. doi: 10.1002/14651858.CD002249.pub5. Cochrane Database Syst Rev. 2016. PMID: 27976369 Free PMC article.
Cited by
-
Pharmacokinetic, pharmacodynamic, and clinical aspects of ovulation induction agents: A review of the literature.J Turk Ger Gynecol Assoc. 2017 Mar 15;18(1):48-55. doi: 10.4274/jtgga.2016.0107. J Turk Ger Gynecol Assoc. 2017. PMID: 28506951 Free PMC article.
-
Clomiphene Citrate Treatment Cycle Outcomes of Polycystic Ovary Syndrome Patients Based on Basal High Sensitive C-Reactive Protein Levels: A Cross-Sectional Study.Int J Fertil Steril. 2017 Jan-Mar;10(4):320-326. doi: 10.22074/ijfs.2016.4849. Epub 2016 Nov 1. Int J Fertil Steril. 2017. PMID: 28042411 Free PMC article.
-
Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice.Clinics (Sao Paulo). 2015 Nov;70(11):765-9. doi: 10.6061/clinics/2015(11)09. Clinics (Sao Paulo). 2015. PMID: 26602525 Free PMC article.
-
Effect of follicle size on pregnancy outcomes in patients undergoing first letrozole-intrauterine insemination.Eur J Med Res. 2024 Mar 18;29(1):184. doi: 10.1186/s40001-024-01794-8. Eur J Med Res. 2024. PMID: 38500174 Free PMC article.
References
References to studies included in this review
George 2007 {published data only}
-
- George K, George S, Chandy A, Raju R, Bala S. hCG administration offers no outcome benefit over spontaneous ovulation in anovulatory women treated with clomiphene citrate. Fertility and Sterility 2007;87(4):985‐7. - PubMed
Yilmaz 2006 {published data only}
-
- Yilmaz B, Kelekci S, Savan K, Oral H, Mollamahmutoglu L. Addition of human chorionic gonadotropin to clomiphene citrate ovulation induction therapy does not improve pregnancy outcomes and luteal function. Fertility and Sterility 2006;85(3):783‐6. - PubMed
References to studies excluded from this review
Harrison 1983 {published data only}
-
- Harrison RF, O'Moorey RR. The use of clomiphene citrate with and without human chorionic gonadotrophin. Irish Medical Journal 1983;76:273‐4. - PubMed
Sutaria 1980 {published data only}
-
- Sutaria UD, Crooke AC, Bertrand PV, Hodgson C. Clomiphene citrate and human chorionic gonadotropin in the treatment of anovulatory infertility. International Journal of Gynaecology and Obstetrics 1980;18:435‐7. - PubMed
Additional references
ASRM 2006
-
- Practice Committee of the American Society of Reproductive Medicine. Use of clomiphene citrate in women. Fertility and Sterility 2006;86 Suppl:S187‐93. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Barbieri 2004
-
- Barbieri RL, Strauss JF. Female infertility. Barbieri RL, Strauss JF, editors .Yen and Jaffe's Reproductive Endocrinology. 5th Edition. Philadelphia: Elsevier Saunders, 2004:644.
Biljan 2005
-
- Billjan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertility and Sterility 2005;84(Suppl 1):S95.
Brown 2009
Coetsier 1996
-
- Coetsier T, Dhont M. Complete and partial luteinized unruptured follicle syndrome after ovarian stimulation with clomiphene citrate/human menopausal gonadotrophin/human chorionic gonadotrophin. Human Reproduction 1996;11:583‐7. - PubMed
Delvigne 2002
-
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update 2002;8(6):559‐77. - PubMed
GRADE 2004 [Computer program]
-
- GRADE Working Group. GRADE Profiler, version 3.6. GRADE Working Group, 2004.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holzer 2006
-
- Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertility and Sterility 2006;85:277‐84. - PubMed
Homburg 2005
-
- Homburg R. Clomiphene citrate ‐ end of an era? A mini review. Human Reproduction 2005;20:2043‐51. - PubMed
ICMART 2006
-
- Zegers‐Hochschild F, Nygren K‐G, Adamson GD, Mouzon J, Lancaster P, Mansour R, et al. The ICMART glossary on ART terminology. Human Reproduction 2006;21(8):1968‐70. - PubMed
Macklon 2004
-
- Fauser BCJM, Macklon NS. Medical approaches to ovarian stimulation for infertility. In: Strauss JF, Barbieri RL editor(s). Yen and Jaffe's Reproductive Endocrinology. 5th Edition. Philadelphia: Elsevier Saunders, 2004:965‐1012.
MECIR 2011
-
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological expectations of Cochrane intervention reviews (MECIR). Methodological standards for the conduct of new Cochrane intervention reviews. Version 2.1, 8 December 2011. http://www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_... (accessed 4 November 2012).
Nargund 2007
-
- Nargund G. Low‐dose hCG is useful in preventing OHSS in high‐risk women without adversely affecting the outcome of IVF cycles. Reproductive Biomedicine Online 2007;14(6):682‐5. - PubMed
NICE 2004
-
- National Collaborating Centre for Women's Health/National Institute for Clinical Excellence. Fertility: Assessment and Treatment for People With Fertility Problems. London: RCOG Press, 2004. - PubMed
Review Manager 5.2 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) Version 5.2. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2012.
Schunemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results, drawing conclusions. Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
Speroff 2005a
-
- Speroff L, Fritz MA. Chapter 31. Induction of ovulation. In: Speroff L, Fritz MA editor(s). Clinical Gynaecologic Endocrinology and Infertility. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, 2005:1175‐214.
Speroff 2005b
-
- Speroff L, Fritz MA. Chapter 27. Female Infertility. In: Speroff L, Fritz MA editor(s). Clinical Gynaecologic Endocrinology and Infertility. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, 2005:1013‐68.
Tulandi 2006
-
- Tulandi T, Martin J, Al‐Fadhli R, Kabli N, Forman R, Hitkari J, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertility and Sterility 2006;85(6):1761‐5. - PubMed
Weil 1999
-
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle stimulating hormone interactions in primate ovarian follicle development. Journal of Clinical Endocrinology and Metabolism 1999;84:2951‐6. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

