Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration
- PMID: 24482234
- PMCID: PMC3953272
- DOI: 10.1074/jbc.M113.517243
Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration
Abstract
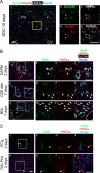
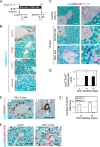
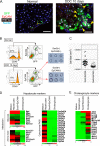
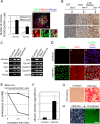
It has been shown that mature hepatocytes compensate tissue damages not only by proliferation and/or hypertrophy but also by conversion into cholangiocyte-like cells. We found that Sry HMG box protein 9-positive (Sox9(+)) epithelial cell adhesion molecule-negative (EpCAM(-)) hepatocyte nuclear factor 4α-positive (HNF4α(+)) biphenotypic cells showing hepatocytic morphology appeared near EpCAM(+) ductular structures in the livers of mice fed 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-containing diet. When Mx1-Cre:ROSA mice, which were injected with poly(I:C) to label mature hepatocytes, were fed with the DDC diet, we found LacZ(+)Sox9(+) cells near ductular structures. Although Sox9(+)EpCAM(-) cells adjacent to expanding ducts likely further converted into ductular cells, the incidence was rare. To know the cellular characteristics of Sox9(+)EpCAM(-) cells, we isolated them as GFP(+)EpCAM(-) cells from DDC-injured livers of Sox9-EGFP mice. Sox9(+)EpCAM(-) cells proliferated and could differentiate to functional hepatocytes in vitro. In addition, Sox9(+)EpCAM(-) cells formed cysts with a small central lumen in collagen gels containing Matrigel® without expressing EpCAM. These results suggest that Sox9(+)EpCAM(-) cells maintaining biphenotypic status can establish cholangiocyte-type polarity. Interestingly, we found that some of the Sox9(+) cells surrounded luminal spaces in DDC-injured liver while they expressed HNF4α. Taken together, we consider that in addition to converting to cholangiocyte-like cells, Sox9(+)EpCAM(-) cells provide luminal space near expanded ductular structures to prevent deterioration of the injuries and potentially supply new hepatocytes to repair damaged tissues.
Keywords: Biphenotypic Cells; Cell Differentiation; Cell Polarity; Ductular Reaction; Hepatocyte; Liver; Liver Stem/Progenitor Cells; Regeneration.
Figures


References
-
- Tanaka M., Itoh T., Tanimizu N., Miyajima A. (2011) Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J. Biochem. 149, 231–239 - PubMed
-
- Cardinale V., Wang Y., Carpino G., Mendel G., Alpini G., Gaudio E., Reid L. M., Alvaro D. (2012) The biliary tree–a reservoir of multipotent stem cells. Nat. Rev. Gastroenterol. Hepatol. 9, 231–240 - PubMed
-
- Okabe M., Tsukahara Y., Tanaka M., Suzuki K., Saito S., Kamiya Y., Tsujimura T., Nakamura K., Miyajima A. (2009) Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 136, 1951–1960 - PubMed
-
- Suzuki A., Sekiya S., Onishi M., Oshima N., Kiyonari H., Nakauchi H., Taniguchi H. (2008) Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology 48, 1964–1978 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

