Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial
- PMID: 24482301
- PMCID: PMC4033144
- DOI: 10.1136/annrheumdis-2013-204655
Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial
Abstract
Objective: Assess ustekinumab efficacy (week 24/week 52) and safety (week 16/week 24/week 60) in patients with active psoriatic arthritis (PsA) despite treatment with conventional and/or biological anti-tumour necrosis factor (TNF) agents.
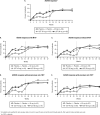
Methods: In this phase 3, multicentre, placebo-controlled trial, 312 adults with active PsA were randomised (stratified by site, weight (≤100 kg/>100 kg), methotrexate use) to ustekinumab 45 mg or 90 mg at week 0, week 4, q12 weeks or placebo at week 0, week 4, week 16 and crossover to ustekinumab 45 mg at week 24, week 28 and week 40. At week 16, patients with <5% improvement in tender/swollen joint counts entered blinded early escape (placebo→45 mg, 45 mg→90 mg, 90 mg→90 mg). The primary endpoint was ≥20% improvement in American College of Rheumatology (ACR20) criteria at week 24. Secondary endpoints included week 24 Health Assessment Questionnaire-Disability Index (HAQ-DI) improvement, ACR50, ACR70 and ≥75% improvement in Psoriasis Area and Severity Index (PASI75). Efficacy was assessed in all patients, anti-TNF-naïve (n=132) patients and anti-TNF-experienced (n=180) patients.
Results: More ustekinumab-treated (43.8% combined) than placebo-treated (20.2%) patients achieved ACR20 at week 24 (p<0.001). Significant treatment differences were observed for week 24 HAQ-DI improvement (p<0.001), ACR50 (p≤0.05) and PASI75 (p<0.001); all benefits were sustained through week 52. Among patients previously treated with ≥1 TNF inhibitor, sustained ustekinumab efficacy was also observed (week 24 combined vs placebo: ACR20 35.6% vs 14.5%, PASI75 47.1% vs 2.0%, median HAQ-DI change -0.13 vs 0.0; week 52 ustekinumab-treated: ACR20 38.9%, PASI75 43.4%, median HAQ-DI change -0.13). No unexpected adverse events were observed through week 60.
Conclusions: The interleukin-12/23 inhibitor ustekinumab (45/90 mg q12 weeks) yielded significant and sustained improvements in PsA signs/symptoms in a diverse population of patients with active PsA, including anti-TNF-experienced PsA patients.
Keywords: Anti-TNF; Psoriatic Arthritis; Spondyloarthritis.
Figures

References
-
- Ritchlin CT. From skin to bone: translational perspectives on psoriatic disease. J Rheumatol 2008;35:1434–7 - PubMed
-
- Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, et al. Psoriatic arthritis (PsA): a clinical, immunological and radiological study of 180 Patients. Br J Rheumatol 1991;30:245–50 - PubMed
-
- Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001;28:1842–6 - PubMed
-
- Wong K, Gladman DD, Husted J, et al. Mortality studies in psoriatic arthritis. Results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum 1997;40:1868–72 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

