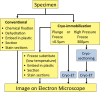
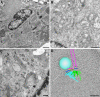
Conventional transmission electron microscopy
- PMID: 24482357
- PMCID: PMC3907272
- DOI: 10.1091/mbc.E12-12-0863
Conventional transmission electron microscopy
Abstract
Researchers have used transmission electron microscopy (TEM) to make contributions to cell biology for well over 50 years, and TEM continues to be an important technology in our field. We briefly present for the neophyte the components of a TEM-based study, beginning with sample preparation through imaging of the samples. We point out the limitations of TEM and issues to be considered during experimental design. Advanced electron microscopy techniques are listed as well. Finally, we point potential new users of TEM to resources to help launch their project.
Figures
References
-
- Bouchet-Marquis C, Hoenger A. Cryo-electron tomography on vitrified sections: a critical analysis of benefits and limitations for structural cell biology. Micron. 2011;42:152–162. - PubMed
-
- Bozzola JJ, Russell LD. Electron Biology Principles and Techniques for Biologists. Sudbury, MA: Jones & Bartlett Learning; 1999.
-
- Dahl R, Staehelin LA. High-pressure freezing for the preservation of biological structure: theory and practice. J Electron Microsc Tech. 1989;13:165–174. - PubMed
-
- Gilkey JC, Staehelin LA. Advances in ultrarapid freezing for the preservation of cellular ultrastructure. J Electron Microsc Tech. 1986;3:177–210.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

