Longitudinal confocal microscopy imaging of solid tumor destruction following adoptive T cell transfer
- PMID: 24482750
- PMCID: PMC3895414
- DOI: 10.4161/onci.26677
Longitudinal confocal microscopy imaging of solid tumor destruction following adoptive T cell transfer
Abstract
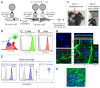
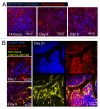
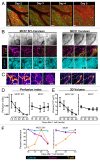
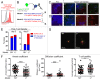
A fluorescence-based, high-resolution imaging approach was used to visualize longitudinally the cellular events unfolding during T cell-mediated tumor destruction. The dynamic interplay of T cells, cancer cells, cancer antigen loss variants, and stromal cells-all color-coded in vivo-was analyzed in established, solid tumors that had developed behind windows implanted on the backs of mice. Events could be followed repeatedly within precisely the same tumor region-before, during and after adoptive T cell therapy-thereby enabling for the first time a longitudinal in vivo evaluation of protracted events, an analysis not possible with terminal imaging of surgically exposed tumors. T cell infiltration, stromal interactions, and vessel destruction, as well as the functional consequences thereof, including the elimination of cancer cells and cancer cell variants were studied. Minimal perivascular T cell infiltrates initiated vascular destruction inside the tumor mass eventually leading to macroscopic central tumor necrosis. Prolonged engagement of T cells with tumor antigen-crosspresenting stromal cells correlated with high IFNγ cytokine release and bystander elimination of antigen-negative cancer cells. The high-resolution, longitudinal, in vivo imaging approach described here will help to further a better mechanistic understanding of tumor eradication by T cells and other anti-cancer therapies.
Keywords: CD8 T cell; cancer; imaging; stroma; tumor immunology; tumor microenvironment.
Figures


References
-
- Seung LP, Seung SK, Schreiber H. Antigenic cancer cells that escape immune destruction are stimulated by host cells. Cancer Res. 1995;55:5094–100. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
