Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells
- PMID: 24482751
- PMCID: PMC3894244
- DOI: 10.4161/onci.26840
Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells
Abstract
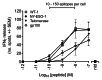
Peptide-pulsed T2 cells are routinely used to study T-cell activation by MHC-restricted peptides derived from tumor-associated antigens (TAAs). Nevertheless, the capacity of T2 cells to present antigenic epitopes remains to be precisely quantified, primarily due to the detection limits imposed by available methods. Since naturally-processed TAA-derived epitopes have been shown to be displayed at levels as low as 10-150 copies per cell, highly sensitive detection and quantification techniques are essential to assess the natural degree of T-cell sensitivity. Here, we report the use of soluble, high-affinity T-cell receptors (TCRs) coupled with single-molecule fluorescence microscopy to quantify three reported TAA-derived epitopes on peptide-pulsed T2 cells, dissecting the relationship between concentration of exogenous peptide, number of epitopes presented, and activation of epitope-specific T cells. Our findings indicate that peptide concentrations in the low nanomolar range are required for T2 cells to present TAAs in extents that are comparable to those of malignant cells.
Keywords: T-cell activation; T-cell receptor; T2 cells; antigen presentation; fluorescence microscopy; monoclonal TCR; tumor-associated antigens.
Figures



References
-
- Hosken NA, Bevan MJ. . Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science 1990; 248:367 - 70; http://dx.doi.org/10.1126/science.2326647; PMID: 2326647 - DOI - PubMed
-
- Purbhoo MA, Sutton DH, Brewer JE, Mullings RE, Hill ME, Mahon TM, Karbach J, Jäger E, Cameron BJ, Lissin N, et al. . . Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol 2006; 176:7308 - 16; PMID: 16751374 - PubMed
-
- Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J, Bianchi FC, Pumphrey NJ, Ladell K, et al. . . Monoclonal TCR-redirected tumor cell killing. Nat Med 2012; 18:980 - 7; http://dx.doi.org/10.1038/nm.2764; PMID: 22561687 - DOI - PubMed
-
- Cohen CJ, Hoffmann N, Farago M, Hoogenboom HR, Eisenbach L, Reiter Y. . Direct detection and quantitation of a distinct T-cell epitope derived from tumor-specific epithelial cell-associated mucin using human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Cancer Res 2002; 62:5835 - 44; PMID: 12384546 - PubMed
-
- Michaeli Y, Denkberg G, Sinik K, Lantzy L, Chih-Sheng C, Beauverd C, Ziv T, Romero P, Reiter Y. . Expression hierarchy of T cell epitopes from melanoma differentiation antigens: unexpected high level presentation of tyrosinase-HLA-A2 Complexes revealed by peptide-specific, MHC-restricted, TCR-like antibodies. J Immunol 2009; 182:6328 - 41; http://dx.doi.org/10.4049/jimmunol.0801898; PMID: 19414786 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
