S-layers: principles and applications
- PMID: 24483139
- PMCID: PMC4232325
- DOI: 10.1111/1574-6976.12063
S-layers: principles and applications
Abstract
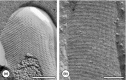
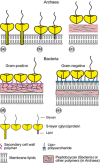
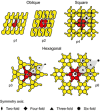
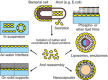
Monomolecular arrays of protein or glycoprotein subunits forming surface layers (S-layers) are one of the most commonly observed prokaryotic cell envelope components. S-layers are generally the most abundantly expressed proteins, have been observed in species of nearly every taxonomical group of walled bacteria, and represent an almost universal feature of archaeal envelopes. The isoporous lattices completely covering the cell surface provide organisms with various selection advantages including functioning as protective coats, molecular sieves and ion traps, as structures involved in surface recognition and cell adhesion, and as antifouling layers. S-layers are also identified to contribute to virulence when present as a structural component of pathogens. In Archaea, most of which possess S-layers as exclusive wall component, they are involved in determining cell shape and cell division. Studies on structure, chemistry, genetics, assembly, function, and evolutionary relationship of S-layers revealed considerable application potential in (nano)biotechnology, biomimetics, biomedicine, and synthetic biology.
Keywords: bacterial surface layers; biomimetics; crystalline cell surface layers (S-layers); nanobiotechnology; self-assembly; synthetic biology.
© 2014 Federation of European Microbiological Societies. Published by John Wiley & Sons Ltd. All rights reserved.
Figures


References
-
- Abdul Halim MF, Pfeiffer F, Zou J et al (2013) Haloferax volcanii archaeosortase is required for motility, mating, and C‐terminal processing of the S‐layer glycoprotein. Mol Microbiol 88: 1164–1175. - PubMed
-
- Akca E, Claus H, Schultz N, Karbach G, Schlott B, Debaerdemaeker T, Declercq JP & König H (2002) Genes and derived amino acid sequences of S‐layer proteins from mesophilic, thermophilic, and extremely thermophilic methanococci. Extremophiles 6: 351–358. - PubMed
-
- Albers SV & Meyer BH (2011) The archaeal cell envelope. Nat Rev Microbiol 9: 414–426. - PubMed
-
- Amselem S, Yogev A, Zawoznik E & Friedman D (1994) Emulsomes, a novel drug delivery technology. Proc Contr Rel Soc 21: 668–669.
-
- Andresen TL, Jensen SS & Jørgensen K (2005) Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res 44: 68–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

