Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae
- PMID: 24483210
- PMCID: PMC4238866
- DOI: 10.1111/1574-6976.12065
Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae
Abstract
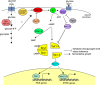
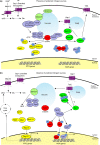
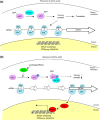
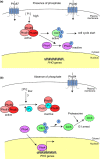
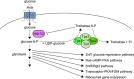
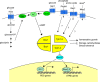
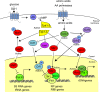
The yeast Saccharomyces cerevisiae has been a favorite organism for pioneering studies on nutrient-sensing and signaling mechanisms. Many specific nutrient responses have been elucidated in great detail. This has led to important new concepts and insight into nutrient-controlled cellular regulation. Major highlights include the central role of the Snf1 protein kinase in the glucose repression pathway, galactose induction, the discovery of a G-protein-coupled receptor system, and role of Ras in glucose-induced cAMP signaling, the role of the protein synthesis initiation machinery in general control of nitrogen metabolism, the cyclin-controlled protein kinase Pho85 in phosphate regulation, nitrogen catabolite repression and the nitrogen-sensing target of rapamycin pathway, and the discovery of transporter-like proteins acting as nutrient sensors. In addition, a number of cellular targets, like carbohydrate stores, stress tolerance, and ribosomal gene expression, are controlled by the presence of multiple nutrients. The protein kinase A signaling pathway plays a major role in this general nutrient response. It has led to the discovery of nutrient transceptors (transporter receptors) as nutrient sensors. Major shortcomings in our knowledge are the relationship between rapid and steady-state nutrient signaling, the role of metabolic intermediates in intracellular nutrient sensing, and the identity of the nutrient sensors controlling cellular growth.
Keywords: G-protein-coupled receptor; Pho85 protein kinase; Ras; Snf1 protein kinase; target of rapamycin; transceptor.
© 2014 The Authors. FEMS Microbiology Reviews published by John Wiley & Sons Ltd on behalf of Federation of European Microbiological Societies.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

