Increased cortical extracellular adenosine correlates with seizure termination
- PMID: 24483230
- PMCID: PMC4104491
- DOI: 10.1111/epi.12511
Increased cortical extracellular adenosine correlates with seizure termination
Erratum in
- Epilepsia. 2014 Sep;55(9):1475
Abstract
Objective: Seizures are currently defined by their electrographic features. However, neuronal networks are intrinsically dependent on neurotransmitters of which little is known regarding their periictal dynamics. Evidence supports adenosine as having a prominent role in seizure termination, as its administration can terminate and reduce seizures in animal models. Furthermore, microdialysis studies in humans suggest that adenosine is elevated periictally, but the relationship to the seizure is obscured by its temporal measurement limitations. Because electrochemical techniques can provide vastly superior temporal resolution, we test the hypothesis that extracellular adenosine concentrations rise during seizure termination in an animal model and humans using electrochemistry.
Methods: White farm swine (n = 45) were used in an acute cortical model of epilepsy, and 10 human epilepsy patients were studied during intraoperative electrocorticography (ECoG). Wireless Instantaneous Neurotransmitter Concentration Sensor (WINCS)-based fast scan cyclic voltammetry (FSCV) and fixed potential amperometry were obtained utilizing an adenosine-specific triangular waveform or biosensors, respectively.
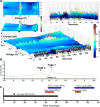
Results: Simultaneous ECoG and electrochemistry demonstrated an average adenosine increase of 260% compared to baseline, at 7.5 ± 16.9 s with amperometry (n = 75 events) and 2.6 ± 11.2 s with FSCV (n = 15 events) prior to electrographic seizure termination. In agreement with these animal data, adenosine elevation prior to seizure termination in a human patient utilizing FSCV was also seen.
Significance: Simultaneous ECoG and electrochemical recording supports the hypothesis that adenosine rises prior to seizure termination, suggesting that adenosine itself may be responsible for seizure termination. Future work using intraoperative WINCS-based FSCV recording may help to elucidate the precise relationship between adenosine and seizure termination.
Keywords: Adenosine; Amperometry; Electrochemistry; Epilepsy; Fast scan cyclic voltammetry; Human trial; Large animal model; Purine; Seizure.
Wiley Periodicals, Inc. © 2014 International League Against Epilepsy.
Conflict of interest statement
Figures




References
-
- Boison D. Adenosine as a modulator of brain activity. Drug News & Perspectives. 2007;20:607–11. - PubMed
-
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends in Pharmacological Sciences. 2006;27:652–8. - PubMed
-
- Aden U, O’Connor WT, Berman RF. Changes in purine levels and adenosine receptors in kindled seizures in the rat. Neuroreport. 2004;15:1585–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

