Obestatin plays an opposite role in the regulation of pituitary somatotrope and corticotrope function in female primates and male/female mice
- PMID: 24484169
- PMCID: PMC3959609
- DOI: 10.1210/en.2013-1728
Obestatin plays an opposite role in the regulation of pituitary somatotrope and corticotrope function in female primates and male/female mice
Abstract
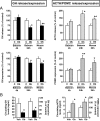
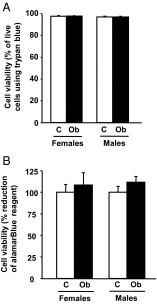
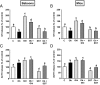
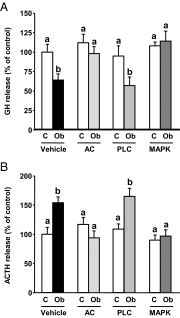
Obestatin is a 23-amino-acid amidated peptide that is encoded by the ghrelin gene. Previous studies have shown obestatin can modulate the hypothalamic neuronal circuitry that regulates pituitary function, perhaps by modulating the actions of ghrelin. However, the direct actions of obestatin on pituitary function remain controversial. Here, primary pituitary cell cultures from a nonhuman primate (baboon) and mice were used to test the effects of obestatin on pituitary hormone expression and secretion. In pituitary cultures from both species, obestatin had no effect on prolactin, LH, FSH, or TSH expression/release. Conversely, obestatin stimulated proopiomelanocortin expression and ACTH release and inhibited GH expression/release in vitro, actions that were also observed in vivo in mice treated with obestatin. In vitro, obestatin inhibited the stimulatory actions of ghrelin on GH but not ACTH release. The inhibitory effect of obestatin on somatotrope function was associated with an overall reduction in pituitary transcription factor-1 and GHRH receptor mRNA levels in vitro and in vivo as well as a reduction in hypothalamic GHRH and ghrelin expression in vivo. The stimulatory effect of obestatin on ACTH was associated with an increase in pituitary CRF receptors. Obestatin also reduced the expression of pituitary somatostatin receptors (sst1/sst2), which could serve to modify its impact on hormone secretion. The in vitro actions of obestatin on both GH and ACTH release required the adenylyl cyclase and MAPK routes. Taken together, our results provide evidence that obestatin can act directly at the pituitary to control somatotrope and corticotrope function, and these effects are conserved across species.
Figures

References
-
- Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310(5750):996–999 - PubMed
-
- Lanfranco F, Motta G, Baldi M, et al. Ghrelin and anterior pituitary function. Front Horm Res. 2010;38:206–211 - PubMed
-
- Granata R, Settanni F, Gallo D, et al. Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes. 2008;57(4):967–979 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

