Parasite clearance following treatment with sulphadoxine-pyrimethamine for intermittent preventive treatment in Burkina-Faso and Mali: 42-day in vivo follow-up study
- PMID: 24484467
- PMCID: PMC3914849
- DOI: 10.1186/1475-2875-13-41
Parasite clearance following treatment with sulphadoxine-pyrimethamine for intermittent preventive treatment in Burkina-Faso and Mali: 42-day in vivo follow-up study
Abstract
Background: Intermittent Preventive Treatment in pregnancy (IPTp) with sulphadoxine-pyrimethamine (SP) is widely used for the control of malaria in pregnancy in Africa. The emergence of resistance to SP is a concern requiring monitoring the effectiveness of SP for IPTp.
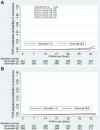
Methods: This was an in-vivo efficacy study to determine the parasitological treatment response and the duration of post-treatment prophylaxis among asymptomatic pregnant women receiving SP as part of IPTp in Mali and Burkina-Faso. The primary outcome was the PCR-unadjusted % of patients with parasites recurrence by day 42 defined as a positive diagnostic test by malaria smear at any visit between days 4 and 42. Treatment failure was based on the standard World Health Organization criteria. The therapeutic response was estimated using the Kaplan-Meier curve.
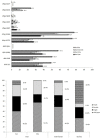
Results: A total of 580 women were enrolled in Mali (N=268) and Burkina-Faso (N=312) and followed weekly for 42 days. Among these, 94.3% completed the follow-up. The PCR-unadjusted cumulative risk of recurrence by day 42 was 4.9% overall, and 3.2% and 6.5% in Mali and Burkina Faso respectively (Hazard Ratio [HR] =2.14, 95%, CI [0.93-4.90]; P=0.070), and higher among the primi- and secundigravida (6.4%) than multigravida (2.2%, HR=3.01 [1.04-8.69]; P=0.042). The PCR-adjusted failure risk was 1.1% overall (Mali 0.8%, Burkina-Faso 1.4%). The frequencies (95% CI) of the dhfr double and triple mutant and dhps 437 and 540 alleles mutant genotype at enrolment were 24.2% (23.7-25.0), 4.7% (4.4-5.0), and 21.4% (20.8-22.0) and 0.37% (0.29-0.44) in Mali, and 7.1% (6.5-7.7), 44.9% (43.8-46.0) and 75.3% (74.5-76.2) and 0% in Burkina-Faso, respectively. There were no dhfr 164L or dhps 581G mutations.
Conclusion: SP remains effective at clearing existing infections when provided as IPTp to asymptomatic pregnant women in Mali and Burkina. Continued monitoring of IPTp-SP effectiveness, including of the impact on birth parameters in this region is essential.
Figures



References
-
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. - PubMed
-
- World Health Organization. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: World Health Organization: Regional Office for Africa; 2004.
-
- Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, MacArthur JR, Luntamo M, Ashorn P, Doumbo OK, ter Kuile FO. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

