Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis
- PMID: 24484547
- PMCID: PMC3937164
- DOI: 10.1186/1479-5876-12-32
Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis
Abstract
Background: Parenterally administered ascorbic acid modulates sepsis-induced inflammation and coagulation in experimental animal models. The objective of this randomized, double-blind, placebo-controlled, phase I trial was to determine the safety of intravenously infused ascorbic acid in patients with severe sepsis.
Methods: Twenty-four patients with severe sepsis in the medical intensive care unit were randomized 1:1:1 to receive intravenous infusions every six hours for four days of ascorbic acid: Lo-AscA (50 mg/kg/24 h, n = 8), or Hi-AscA (200 mg/kg/24 h, n = 8), or Placebo (5% dextrose/water, n = 8). The primary end points were ascorbic acid safety and tolerability, assessed as treatment-related adverse-event frequency and severity. Patients were monitored for worsened arterial hypotension, tachycardia, hypernatremia, and nausea or vomiting. In addition Sequential Organ Failure Assessment (SOFA) scores and plasma levels of ascorbic acid, C-reactive protein, procalcitonin, and thrombomodulin were monitored.
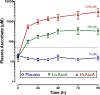
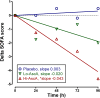
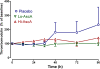
Results: Mean plasma ascorbic acid levels at entry for the entire cohort were 17.9 ± 2.4 μM (normal range 50-70 μM). Ascorbic acid infusion rapidly and significantly increased plasma ascorbic acid levels. No adverse safety events were observed in ascorbic acid-infused patients. Patients receiving ascorbic acid exhibited prompt reductions in SOFA scores while placebo patients exhibited no such reduction. Ascorbic acid significantly reduced the proinflammatory biomarkers C-reactive protein and procalcitonin. Unlike placebo patients, thrombomodulin in ascorbic acid infused patients exhibited no significant rise, suggesting attenuation of vascular endothelial injury.
Conclusions: Intravenous ascorbic acid infusion was safe and well tolerated in this study and may positively impact the extent of multiple organ failure and biomarkers of inflammation and endothelial injury.
Trial registration: ClinicalTrials.gov identifier NCT01434121.
Figures

References
-
- Quenot JP, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V, Barraud D, Cousson J, Louis G, Perez P, Kuteifan K, Noirot A, Badie J, Mezher C, Lessire H, Pavon A, Study Group E. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care. 2013;17(2):R65. doi: 10.1186/cc12598. doi: 10.1186/cc12598. PMID: 23561510. - DOI - PMC - PubMed
-
- Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, Michalopoulos A, Vincent JL. The SOAP Investigators. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16(6):R222. doi: 10.1186/cc11868. doi: 10.1186/cc11868. PMID: 23158219. - DOI - PMC - PubMed
-
- Marshall JC. Clinical trials of mediator-directed therapy in sepsis: What have we learned? Intensive Care Med. 2000;26(Suppl 1):S75–S83. doi: 10.1007/s001340051122. PMID: 10786962. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

