Dihydrotestosterone potentiates EGF-induced ERK activation by inducing SRC in fetal lung fibroblasts
- PMID: 24484548
- PMCID: PMC4091851
- DOI: 10.1165/rcmb.2012-0179OC
Dihydrotestosterone potentiates EGF-induced ERK activation by inducing SRC in fetal lung fibroblasts
Abstract
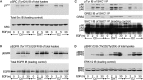
Lung maturation is regulated by interactions between mesenchymal and epithelial cells, and is delayed by androgens. Fibroblast-Type II cell communications are dependent on extracellular signal-regulated kinases (ERK) 1/2 activation by the ErbB receptor ligands epidermal growth factor (EGF), transforming growth factor (TGF)-α, and neuregulin (Nrg). In other tissues, dihydrotestosterone (DHT) has been shown to activate SRC by a novel nontranscriptional mechanism, which phosphorylates EGF receptors to potentiate EGF-induced ERK1/2 activation. This study sought to determine if DHT potentiates EGFR signaling by a nontranscriptional mechanism. Embryonic day (E)17 fetal lung cells were isolated from dams treated with or without DHT since E12. Cells were exposed to 30 ng/ml DHT for periods of 30 minutes to 3 days before being stimulated with 100 ng/ml EGF, TGF-α, or Nrg for up to 30 minutes. Lysates were immunoblotted for ErbB and SRC pathway signaling intermediates. DHT increased ERK1/2 activation by EGF, TGF-α, and Nrg in fibroblasts and Type II cells. Characterization in fibroblasts showed that potentiation of the EGF pathway was significant after 60 minutes of DHT exposure and persisted in the presence of the translational inhibitor cycloheximide. SRC and EGF receptor phosphorylation was increased by DHT, as was EGF-induced SHC1 phosphorylation and subsequent association with GRB2. Finally, SRC silencing, SRC inhibition with PP2, and overexpression of a dominant-negative SRC each prevented DHT from increasing EGF-induced ERK1/2 phosphorylation. These results suggest that DHT activates SRC to potentiate the signaling pathway leading from the EGF receptor to ERK activation in primary fetal lung fibroblasts.
Keywords: ErbB; MAP kinases; androgens; fibroblasts.
Figures




References
-
- Torday JS, Nielsen HC, Fencl MM, Avery ME. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981;123:205–208. - PubMed
-
- Miller HC, Futrakul P. Birth weight, gestational age, and sex as determining factors in the incidence of respiratory distress syndrome of prematurely born infants. J Pediatr. 1968;72:628–635. - PubMed
-
- Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–688. - PubMed
-
- Gross I, Wilson CM, Ingleson LD, Brehier A, Rooney SA. The influence of hormones on the biochemical development of fetal rat lung in organ culture: I. Estrogen. Biochim Biophys Acta. 1979;575:375–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

