miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2
- PMID: 24484937
- PMCID: PMC5528633
- DOI: 10.1016/j.molonc.2014.01.005
miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2
Abstract
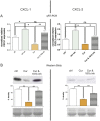
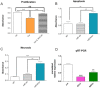
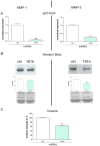
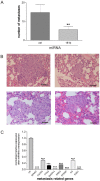
Chronic inflammation is a major risk factor for the development and metastatic progression of cancer. We have previously reported that the chemopreventive polyphenol Curcumin inhibits the expression of the proinflammatory cytokines CXCL1 and -2 leading to diminished formation of breast and prostate cancer metastases. In the present study, we have analyzed the effects of Curcumin on miRNA expression and its correlation to the anti-tumorigenic properties of this natural occurring polyphenol. Using microarray miRNA expression analyses, we show here that Curcumin modulates the expression of a series of miRNAs, including miR181b, in metastatic breast cancer cells. Interestingly, we found that miR181b down-modulates CXCL1 and -2 through a direct binding to their 3'-UTR. Overexpression or inhibition of miR181b in metastatic breast cancer cells has a significant impact on CXCL1 and -2 and is required for the effect of Curcumin on these two cytokines. miR181b also mediates the effects of Curcumin on inhibition of proliferation and invasion as well as induction of apoptosis. Importantly, over-expression of miR181b in metastatic breast cancer cells inhibits metastasis formation in vivo in immunodeficient mice. Finally, we demonstrated that Curcumin up-regulates miR181b and down-regulates CXCL1 and -2 in cells isolated from several primary human breast cancers. Taken together, these data show that Curcumin provides a simple bridge to bring metastamir modulation into the clinic, placing it in a primary and tertiary preventive, as well as a therapeutic, setting.
Keywords: Breast cancer; Curcumin; Inflammatory cytokines; Metastases prevention; microRNAs.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Albini, A., Indraccolo, S., Noonan, D.M., Pfeffer, U., Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs. Clin. Exp. Metastasis 27, 419–439. - PubMed
-
- Albini, A. , Mirisola, V. , Pfeffer, U. , 2008. Metastasis signatures: genes regulating tumor-microenvironment interactions predict metastatic behavior. Cancer Metastasis Rev.. 27, 75–83. - PubMed
-
- Albini, A. , Sporn, M.B. , 2007. The tumour microenvironment as a target for chemoprevention. Nat.Rev. Cancer. 7, 139–147. - PubMed
-
- Albini, A. , Tosetti, F. , Benelli, R. , Noonan, D.M. , 2005. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res.. 65, 10637–10641. - PubMed
-
- Albini, A. , Tosetti, F. , Li, V.W. , Noonan, D.M. , Li, W.W. , 2012. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol.. 9, 498–509. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

