Nicotinic receptor antagonists as treatments for nicotine abuse
- PMID: 24484986
- PMCID: PMC4110698
- DOI: 10.1016/B978-0-12-420118-7.00013-5
Nicotinic receptor antagonists as treatments for nicotine abuse
Abstract
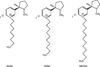
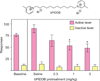
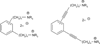
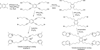
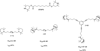
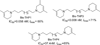
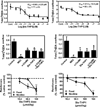
Despite the proven efficacy of current pharmacotherapies for tobacco dependence, relapse rates continue to be high, indicating that novel medications are needed. Currently, several smoking cessation agents are available, including varenicline (Chantix®), bupropion (Zyban®), and cytisine (Tabex®). Varenicline and cytisine are partial agonists at the α4β2* nicotinic acetylcholine receptor (nAChR). Bupropion is an antidepressant but is also an antagonist at α3β2* ganglionic nAChRs. The rewarding effects of nicotine are mediated, in part, by nicotine-evoked dopamine (DA) release leading to sensitization, which is associated with repeated nicotine administration and nicotine addiction. Receptor antagonists that selectivity target central nAChR subtypes mediating nicotine-evoked DA release should have efficacy as tobacco use cessation agents with the therapeutic advantage of a limited side-effect profile. While α-conotoxin MII (α-CtxMII)-insensitive nAChRs (e.g., α4β2*) contribute to nicotine-evoked DA release, these nAChRs are widely distributed in the brain, and inhibition of these receptors may lead to nonselective and untoward effects. In contrast, α-CtxMII-sensitive nAChRs mediating nicotine-evoked DA release offer an advantage as targets for smoking cessation, due to their more restricted localization primarily to dopaminergic neurons. Small drug-like molecules that are selective antagonists at α-CtxMII-sensitive nAChR subtypes that contain α6 and β2 subunits have now been identified. Early research identified a variety of quaternary ammonium analogs that were potent and selective antagonists at nAChRs mediating nicotine-evoked DA release. More recent data have shown that novel, nonquaternary bis-1,2,5,6-tetrahydropyridine analogs potently inhibit (IC50<1nM) nicotine-evoked DA release in vitro by acting as antagonists at α-CtxMII-sensitive nAChR subtypes; these compounds also decrease NIC self-administration in rats.
Keywords: Nicotine analogs; Nicotine dependence; Nicotinic receptor antagonists; Relapse; Smoking cessation.
© 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
A potential royalty stream to PAC and LPD may occur consistent with University of Kentucky policy.
Figures





Similar articles
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
Nicotinic receptor-based therapeutics and candidates for smoking cessation.Biochem Pharmacol. 2009 Oct 1;78(7):732-43. doi: 10.1016/j.bcp.2009.06.002. Epub 2009 Jun 10. Biochem Pharmacol. 2009. PMID: 19523455 Free PMC article. Review.
-
Differential modulation of brain nicotinic acetylcholine receptor function by cytisine, varenicline, and two novel bispidine compounds: emergent properties of a hybrid molecule.J Pharmacol Exp Ther. 2013 Nov;347(2):424-37. doi: 10.1124/jpet.113.206904. Epub 2013 Aug 19. J Pharmacol Exp Ther. 2013. PMID: 23959137 Free PMC article.
-
Repeated nicotine administration robustly increases bPiDDB inhibitory potency at alpha6beta2-containing nicotinic receptors mediating nicotine-evoked dopamine release.Biochem Pharmacol. 2010 Aug 1;80(3):402-9. doi: 10.1016/j.bcp.2010.03.018. Epub 2010 Mar 25. Biochem Pharmacol. 2010. PMID: 20346923 Free PMC article.
-
N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity.J Pharmacol Exp Ther. 2008 Aug;326(2):563-76. doi: 10.1124/jpet.108.136630. Epub 2008 May 6. J Pharmacol Exp Ther. 2008. PMID: 18460644 Free PMC article.
Cited by
-
Role of corticotropin-releasing factor in alcohol and nicotine addiction.Brain Res. 2020 Aug 1;1740:146850. doi: 10.1016/j.brainres.2020.146850. Epub 2020 Apr 21. Brain Res. 2020. PMID: 32330519 Free PMC article. Review.
-
Efficient expression of functional (α6β2)2β3 AChRs in Xenopus oocytes from free subunits using slightly modified α6 subunits.PLoS One. 2014 Jul 28;9(7):e103244. doi: 10.1371/journal.pone.0103244. eCollection 2014. PLoS One. 2014. PMID: 25068303 Free PMC article.
-
Rodent models for nicotine withdrawal.J Psychopharmacol. 2021 Oct;35(10):1169-1187. doi: 10.1177/02698811211005629. Epub 2021 Apr 22. J Psychopharmacol. 2021. PMID: 33888006 Free PMC article. Review.
-
Synthesis and Pharmacological Evaluation of DHβE Analogues as Neuronal Nicotinic Acetylcholine Receptor Antagonists.ACS Med Chem Lett. 2014 May 20;5(7):766-70. doi: 10.1021/ml500094c. eCollection 2014 Jul 10. ACS Med Chem Lett. 2014. PMID: 25050162 Free PMC article.
-
r-bPiDI, an α6β2* Nicotinic Receptor Antagonist, Decreases Nicotine-Evoked Dopamine Release and Nicotine Reinforcement.Neurochem Res. 2015 Oct;40(10):2121-30. doi: 10.1007/s11064-015-1680-4. Epub 2015 Jul 31. Neurochem Res. 2015. PMID: 26227997 Free PMC article.
References
-
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. Journal of Biological Chemistry. 1991;266:11192–11198. - PubMed
-
- Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. bis-Azaaromatic quaternary ammonium analogues: Ligands for α4β2 and α7* subtypes of neuronal nicotinic receptors. Bioorganic and Medicinal Chemistry Letters. 2002;12:3067–3071. - PubMed
-
- Bacher I, Wu B, Shytle DR, George TP. Mecamylamine—A nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opinion on Pharmacotherapy. 2009;10:2709–2721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

