New directions in nicotine vaccine design and use
- PMID: 24484987
- PMCID: PMC4047682
- DOI: 10.1016/B978-0-12-420118-7.00014-7
New directions in nicotine vaccine design and use
Abstract
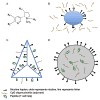
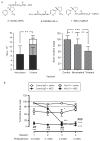
Clinical trials of nicotine vaccines suggest that they can enhance smoking cessation rates but do not reliably produce the consistently high serum antibody concentrations required. A wide array of next-generation strategies are being evaluated to enhance vaccine efficacy or provide antibody through other mechanisms. Protein conjugate vaccines may be improved by modifications of hapten or linker design or by optimizing hapten density. Conjugating hapten to viruslike particles or disrupted virus may allow exploitation of naturally occurring viral features associated with high immunogenicity. Conjugates that utilize different linker positions on nicotine can function as independent immunogens, so that using them in combination generates higher antibody concentrations than can be produced by a single immunogen. Nanoparticle vaccines, consisting of hapten, T cell help peptides, and adjuvants attached to a liposome or synthetic scaffold, are in the early stages of development. Nanoparticle vaccines offer the possibility of obtaining precise and consistent control of vaccine component stoichiometry and spacing and immunogen size and shape. Passive transfer of nicotine-specific monoclonal antibodies offers a greater control of antibody dose, the ability to give very high doses, and an immediate onset of action but is expensive and has a shorter duration of action than vaccines. Viral vector-mediated transfer of genes for antibody production can elicit high levels of antibody expression in animals and may present an alternative to vaccination or passive immunization if the long-term safety of this approach is confirmed. Next-generation immunotherapies are likely to be substantially more effective than first-generation vaccines.
Keywords: Addiction; Immunogen; Immunotherapy; Nicotine; Vaccine.
© 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures

References
-
- Anonymous. The health consequences of smoking: Nicotine addiction, a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health; 1988.
-
- Anonymous. Trial watch: Xenova’s TA-NIC vaccine shows promise. Expert Review of Vaccines. 2004;3:386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

