mTOR signaling in autophagy regulation in the kidney
- PMID: 24485024
- PMCID: PMC4911697
- DOI: 10.1016/j.semnephrol.2013.11.002
mTOR signaling in autophagy regulation in the kidney
Abstract
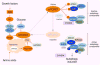
Cells possess adaptive biosynthetic systems to maintain cellular energy levels for survival under adverse environmental conditions. Autophagy is an evolutionarily conserved cellular catabolic process that breaks down and recycles cytosolic material including macromolecules and organelles through lysosomal degradation. This catabolic process, represented by macroautophagy, is induced by a variety of cellular stresses such as nutrient starvation, which causes a shortage of cellular energy for cells to maintain cellular homeostasis and essential biological activities. In contrast, upon nutrient availability, cells stimulate anabolic processes. The mechanistic/mammalian target of rapamycin, a serine/threonine protein kinase, is a key player in stimulating cellular anabolism in response to nutrients and growth factors, and plays a crucial role in suppressing autophagy activity. Growing evidence has suggested that autophagy activity is required for the maintenance and physiological functions of renal cells including proximal tubular cells and podocytes. In this article, we discuss recent progress in the regulation of autophagy by mechanistic/mammalian target of rapamycin signaling.
Keywords: AMPK; Autophagy; mTOR; mTORC1; podocyte; rapamycin; renal proximal tubular cell.
© 2014 Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Statement: None.
Figures
References
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. - PubMed
-
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular cell. 2002;10:457–68. - PubMed
-
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB. 2004;14:1296–302. - PubMed
-
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

