Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit
- PMID: 24485458
- PMCID: PMC3982923
- DOI: 10.1016/j.cell.2013.12.040
Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit
Abstract
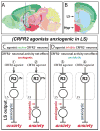
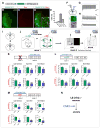
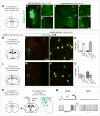
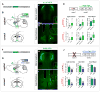
The extended amygdala has dominated research on the neural circuitry of fear and anxiety, but the septohippocampal axis also plays an important role. The lateral septum (LS) is thought to suppress fear and anxiety through its outputs to the hypothalamus. However, this structure has not yet been dissected using modern tools. The type 2 CRF receptor (Crfr2) marks a subset of LS neurons whose functional connectivity we have investigated using optogenetics. Crfr2(+) cells include GABAergic projection neurons that connect with the anterior hypothalamus. Surprisingly, we find that these LS outputs enhance stress-induced behavioral measures of anxiety. Furthermore, transient activation of Crfr2(+) neurons promotes, while inhibition suppresses, persistent anxious behaviors. LS Crfr2(+) outputs also positively regulate circulating corticosteroid levels. These data identify a subset of LS projection neurons that promote, rather than suppress, stress-induced behavioral and endocrinological dimensions of persistent anxiety states and provide a cellular point of entry to LS circuitry.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiology & behavior. 2006;88:12–29. - PubMed
-
- Albert DJ, Richmond SE. Hyperreactivity and aggressiveness following infusion of local anesthetic into the lateral septum or surrounding structures. Behavioral biology. 1976;18:211–226. - PubMed
-
- Albert DJ, Wong RC. Hyperreactivity, muricide, and intraspecific aggression in the rat produced by infusion of local anesthetic into the lateral septum or surrounding areas. J Comp Physiol Psychol. 1978;92:1062–1073. - PubMed
-
- Almeida-Santos AF, Gobira PH, Rosa LC, Guimaraes FS, Moreira FA, Aguiar DC. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav Brain Res. 2013;252:10–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

