Sensitizing protective tumor microenvironments to antibody-mediated therapy
- PMID: 24485462
- PMCID: PMC3975171
- DOI: 10.1016/j.cell.2013.12.041
Sensitizing protective tumor microenvironments to antibody-mediated therapy
Abstract
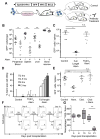
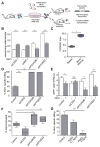
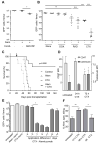
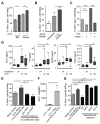
Therapy-resistant microenvironments represent a major barrier toward effective elimination of disseminated malignancies. Here, we show that select microenvironments can underlie resistance to antibody-based therapy. Using a humanized model of treatment refractory B cell leukemia, we find that infiltration of leukemia cells into the bone marrow rewires the tumor microenvironment to inhibit engulfment of antibody-targeted tumor cells. Resistance to macrophage-mediated killing can be overcome by combination regimens involving therapeutic antibodies and chemotherapy. Specifically, the nitrogen mustard cyclophosphamide induces an acute secretory activating phenotype (ASAP), releasing CCL4, IL8, VEGF, and TNFα from treated tumor cells. These factors induce macrophage infiltration and phagocytic activity in the bone marrow. Thus, the acute induction of stress-related cytokines can effectively target cancer cells for removal by the innate immune system. This synergistic chemoimmunotherapeutic regimen represents a potent strategy for using conventional anticancer agents to alter the tumor microenvironment and promote the efficacy of targeted therapeutics.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
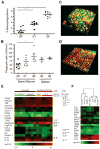
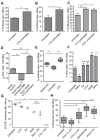
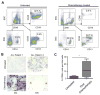
Comment in
-
Resistance: turning macrophages on, off and on again.Nat Rev Cancer. 2014 Mar;14(3):154-5. doi: 10.1038/nrc3688. Nat Rev Cancer. 2014. PMID: 24561433 No abstract available.
-
Tumour immunology: turning macrophages on, off and on again.Nat Rev Immunol. 2014 Mar;14(3):136-7. doi: 10.1038/nri3634. Nat Rev Immunol. 2014. PMID: 24566906 No abstract available.
References
-
- Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, Kluin PM. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. - PubMed
-
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature Medicine. 2000;6:443–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

