Spatial patterning of BMP-2 and BMP-7 on biopolymeric films and the guidance of muscle cell fate
- PMID: 24485790
- PMCID: PMC4155073
- DOI: 10.1016/j.biomaterials.2014.01.012
Spatial patterning of BMP-2 and BMP-7 on biopolymeric films and the guidance of muscle cell fate
Abstract
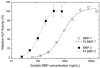
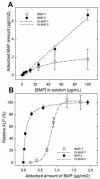
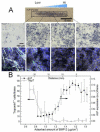
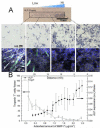
In the cellular microenvironment, growth factor gradients are crucial in dictating cell fate. Towards developing materials that capture the native microenvironment we engineered biomimetic films that present gradients of matrix-bound bone morphogenetic proteins (BMP-2 and BMP-7). To this end layer-by-layer films composed of poly(L-lysine) and hyaluronan were combined in a simple microfluidic device enabling spatially controlled growth factor diffusion along the film. Linear long-range gradients of both BMPs induced the trans-differentiation of C2C12 myoblasts towards the osteogenic lineage in a dose dependent manner with a different signature for each BMP. The osteogenic marker alkaline phosphatase (ALP) increased in a linear manner for BMP-7 and non-linearly for BMP-2. Moreover, an increased expression of the myogenic marker troponin T was observed with decreasing matrix-bound BMP concentration, providing a substrate that it is both osteo- and myo-inductive. Lastly, dual parallel matrix-bound gradients of BMP-2 and -7 revealed a complete saturation of the ALP signal. This suggested an additive or synergistic effect of the two BMPs. This simple technology allows for determining quickly and efficiently the optimal concentration of matrix-bound growth factors, as well as for investigating the presentation of multiple growth factors in their solid-phase and in a spatially controlled manner.
Keywords: Bone morphogenetic proteins; Gradient; Layer-by-layer; Microfluidic; Myogenesis; Osteoinduction.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Stiffness-dependent cellular internalization of matrix-bound BMP-2 and its relation to Smad and non-Smad signaling.Acta Biomater. 2016 Dec;46:55-67. doi: 10.1016/j.actbio.2016.09.014. Epub 2016 Sep 12. Acta Biomater. 2016. PMID: 27633320 Free PMC article.
-
Presentation of BMP-2 from a soft biopolymeric film unveils its activity on cell adhesion and migration.Adv Mater. 2011 Mar 25;23(12):H111-8. doi: 10.1002/adma.201004637. Epub 2011 Feb 25. Adv Mater. 2011. PMID: 21433098 No abstract available.
-
Bone morphogenetic protein-2 functions as a negative regulator in the differentiation of myoblasts, but not as an inducer for the formations of cartilage and bone in mouse embryonic tongue.BMC Dev Biol. 2011 Jul 7;11:44. doi: 10.1186/1471-213X-11-44. BMC Dev Biol. 2011. PMID: 21736745 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine.Vitam Horm. 2015;99:293-322. doi: 10.1016/bs.vh.2015.06.002. Epub 2015 Jul 15. Vitam Horm. 2015. PMID: 26279381 Review.
Cited by
-
Matrix-Immobilized BMP-2 on Microcontact Printed Fibronectin as an in vitro Tool to Study BMP-Mediated Signaling and Cell Migration.Front Bioeng Biotechnol. 2015 May 11;3:62. doi: 10.3389/fbioe.2015.00062. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 26029690 Free PMC article.
-
Bu-M-P-ing Iron: How BMP Signaling Regulates Muscle Growth and Regeneration.J Dev Biol. 2020 Feb 11;8(1):4. doi: 10.3390/jdb8010004. J Dev Biol. 2020. PMID: 32053985 Free PMC article. Review.
-
Cellular Response to Bone Morphogenetic Proteins-2 and -7 Covalently Bound to Photocrosslinked Heparin-Diazoresin Multilayer.Biomolecules. 2023 May 15;13(5):842. doi: 10.3390/biom13050842. Biomolecules. 2023. PMID: 37238712 Free PMC article.
-
Learning from BMPs and their biophysical extracellular matrix microenvironment for biomaterial design.Bone. 2020 Dec;141:115540. doi: 10.1016/j.bone.2020.115540. Epub 2020 Jul 27. Bone. 2020. PMID: 32730925 Free PMC article. Review.
-
Thickness Gradient in Polymer Coating by Reactive Layer-by-Layer Assembly on Solid Substrate.ACS Omega. 2023 Sep 26;8(40):37413-37420. doi: 10.1021/acsomega.3c05445. eCollection 2023 Oct 10. ACS Omega. 2023. PMID: 37841123 Free PMC article.
References
-
- Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol. 2009;19:543–8. - PubMed
-
- Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–21. - PubMed
-
- Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

