The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells
- PMID: 24485793
- PMCID: PMC3982910
- DOI: 10.1016/j.biomaterials.2014.01.037
The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells
Abstract
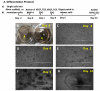
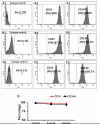
Current EC differentiation protocols are inefficient, and the phenotypes of the differentiated ECs are only briefly stable, which significantly inhibits their utility for basic science research. Here, a remarkably more efficient hiPSC-EC differentiation protocol that incorporates a three-dimensional (3D) fibrin scaffold is presented. With this protocol, up to 45% of the differentiated hiPSCs assumed an EC phenotype, and after purification, greater than 95% of the cells displayed the EC phenotype (based on CD31 expression). The hiPSC-ECs continued to display EC characteristics for 4 weeks in vitro. Gene and protein expression levels of CD31, CD144 and von Willebrand factor-8 (vWF-8) were significantly up-regulated in differentiated hiPSC-ECs. hiPSC-ECs also have biological function to up-take Dil-conjugated acetylated LDL (Dil-ac-LDL) and form tubular structures on Matrigel. Collectively, these data demonstrate that a 3D differentiation protocol can efficiently generate ECs from hiPSCs and, furthermore, the differentiated hiPSC-ECs are functional and can maintain EC fate up to 4 weeks in vitro.
Keywords: Cell differentiation; Eddothelial cells; Human induced pluripotent stem cells; Scaffold.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–7. - PubMed
-
- Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, et al. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol. 2006;291:H648–57. - PubMed
-
- Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, et al. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

