A role for myosin II in mammalian mitochondrial fission
- PMID: 24485837
- PMCID: PMC3958938
- DOI: 10.1016/j.cub.2013.12.032
A role for myosin II in mammalian mitochondrial fission
Abstract
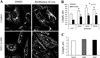
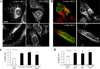
Mitochondria are dynamic organelles, undergoing both fission and fusion regularly in interphase cells. Mitochondrial fission is thought to be part of a quality-control mechanism whereby damaged mitochondrial components are segregated from healthy components in an individual mitochondrion, followed by mitochondrial fission and degradation of the damaged daughter mitochondrion. Fission also plays a role in apoptosis. Defects in mitochondrial dynamics can lead to neurodegenerative diseases such as Alzheimer's disease. Mitochondrial fission requires the dynamin GTPase Drp1, which assembles in a ring around the mitochondrion and appears to constrict both outer and inner mitochondrial membranes. However, mechanisms controlling Drp1 assembly on mammalian mitochondria are unclear. Recent results show that actin polymerization, driven by the endoplasmic reticulum-bound formin protein INF2, stimulates Drp1 assembly at fission sites. Here, we show that myosin II also plays a role in fission. Chemical inhibition by blebbistatin or small interfering RNA (siRNA)-mediated suppression of myosin IIA or myosin IIB causes an increase in mitochondrial length in both control cells and cells expressing constitutively active INF2. Active myosin II accumulates in puncta on mitochondria in an actin- and INF2-dependent manner. In addition, myosin II inhibition decreases Drp1 association with mitochondria. Based on these results, we propose a mechanistic model in which INF2-mediated actin polymerization leads to myosin II recruitment and constriction at the fission site, enhancing subsequent Drp1 accumulation and fission.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

