Surrogate endpoints for overall survival in metastatic melanoma: a meta-analysis of randomised controlled trials
- PMID: 24485879
- PMCID: PMC4443445
- DOI: 10.1016/S1470-2045(14)70007-5
Surrogate endpoints for overall survival in metastatic melanoma: a meta-analysis of randomised controlled trials
Abstract
Background: Recent phase 3 trials have shown an overall survival benefit in metastatic melanoma. We aimed to assess whether progression-free survival (PFS) could be regarded as a reliable surrogate for overall survival through a meta-analysis of randomised trials.
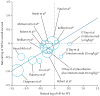
Methods: We systematically reviewed randomised trials comparing treatment regimens in metastatic melanoma that included dacarbazine as the control arm, and which reported both PFS and overall survival with a standard hazard ratio (HR). We correlated HRs for overall survival and PFS, weighted by sample size or by precision of the HR estimate, assuming fixed and random effects. We did sensitivity analyses according to presence of crossover, trial size, and dacarbazine dose.
Findings: After screening 1649 reports and meeting abstracts published before Sept 8, 2013, we identified 12 eligible randomised trials that enrolled 4416 patients with metastatic melanoma. Irrespective of weighting strategy, we noted a strong correlation between the treatment effects for PFS and overall survival, which seemed independent of treatment type. Pearson correlation coefficients were 0·71 (95% CI 0·29-0·90) with a random-effects assumption, 0·85 (0·59-0·95) with a fixed-effects assumption, and 0·89 (0·68-0·97) with sample-size weighting. For nine trials without crossover, the correlation coefficient was 0·96 (0·81-0·99), which decreased to 0·93 (0·74-0·98) when two additional trials with less than 50% crossover were included. Inclusion of mature follow-up data after at least 50% crossover (in vemurafenib and dabrafenib phase 3 trials) weakened the PFS to overall survival correlation (0·55, 0·03-0·84). Inclusion of trials with no or little crossover with the random-effects assumption yielded a conservative statement of the PFS to overall survival correlation of 0·85 (0·51-0·96).
Interpretation: PFS can be regarded as a robust surrogate for overall survival in dacarbazine-controlled randomised trials of metastatic melanoma; we postulate that this association will hold as treatment standards evolve and are adopted as the control arm in future trials.
Funding: None.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
PFS as a surrogate for overall survival in metastatic melanoma.Lancet Oncol. 2014 Mar;15(3):246-8. doi: 10.1016/S1470-2045(14)70039-7. Epub 2014 Jan 31. Lancet Oncol. 2014. PMID: 24485878 No abstract available.
Similar articles
-
Dabrafenib for Treating Unresectable, Advanced or Metastatic BRAF V600 Mutation-Positive Melanoma: An Evidence Review Group Perspective.Pharmacoeconomics. 2015 Sep;33(9):893-904. doi: 10.1007/s40273-015-0276-9. Pharmacoeconomics. 2015. PMID: 25906420 Review.
-
Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial.Lancet. 2012 Jul 28;380(9839):358-65. doi: 10.1016/S0140-6736(12)60868-X. Epub 2012 Jun 25. Lancet. 2012. PMID: 22735384 Clinical Trial.
-
Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study.Lancet Oncol. 2014 Mar;15(3):323-32. doi: 10.1016/S1470-2045(14)70012-9. Epub 2014 Feb 7. Lancet Oncol. 2014. PMID: 24508103 Free PMC article. Clinical Trial.
-
Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study.Lancet Oncol. 2013 Jul;14(8):733-40. doi: 10.1016/S1470-2045(13)70237-7. Epub 2013 Jun 2. Lancet Oncol. 2013. PMID: 23735514 Clinical Trial.
-
Early analysis of surrogate endpoints for metastatic melanoma in immune checkpoint inhibitor trials.Medicine (Baltimore). 2016 Jun;95(26):e3997. doi: 10.1097/MD.0000000000003997. Medicine (Baltimore). 2016. PMID: 27368007 Free PMC article. Review.
Cited by
-
Immune Checkpoint Blockade: The New Frontier in Cancer Treatment.Target Oncol. 2018 Feb;13(1):1-20. doi: 10.1007/s11523-017-0549-7. Target Oncol. 2018. PMID: 29441437
-
Evaluation of classical clinical endpoints as surrogates for overall survival in patients treated with immune checkpoint blockers: a systematic review and meta-analysis.J Cancer Res Clin Oncol. 2018 Nov;144(11):2245-2261. doi: 10.1007/s00432-018-2738-x. Epub 2018 Aug 21. J Cancer Res Clin Oncol. 2018. PMID: 30132118 Free PMC article.
-
The Reporting and Methodological Quality of Systematic Reviews Underpinning Clinical Practice Guidelines Focused on the Management of Cutaneous Melanoma: Cross-Sectional Analysis.JMIR Dermatol. 2023 Dec 7;6:e43821. doi: 10.2196/43821. JMIR Dermatol. 2023. PMID: 38060306 Free PMC article.
-
Era of surrogate endpoints and accelerated approvals: a comprehensive review on applicability, uncertainties, and challenges from regulatory, payer, and patient perspectives.Eur J Clin Pharmacol. 2025 May;81(5):605-623. doi: 10.1007/s00228-025-03822-w. Epub 2025 Mar 13. Eur J Clin Pharmacol. 2025. PMID: 40080138 Review.
-
The incidence and risk of cutaneous toxicities associated with dabrafenib in melanoma patients: a systematic review and meta-analysis.Eur J Hosp Pharm. 2021 Jul;28(4):182-189. doi: 10.1136/ejhpharm-2020-002347. Epub 2020 Sep 3. Eur J Hosp Pharm. 2021. PMID: 32883694 Free PMC article.
References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

