Rad54 functions as a heteroduplex DNA pump modulated by its DNA substrates and Rad51 during D loop formation
- PMID: 24486020
- PMCID: PMC4059524
- DOI: 10.1016/j.molcel.2013.12.027
Rad54 functions as a heteroduplex DNA pump modulated by its DNA substrates and Rad51 during D loop formation
Abstract
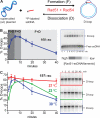
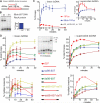
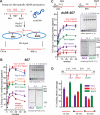
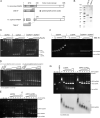
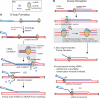
The displacement loop (D loop) is the product of homology search and DNA strand invasion, constituting a central intermediate in homologous recombination (HR). In eukaryotes, the Rad51 DNA strand exchange protein is assisted in D loop formation by the Rad54 motor protein. Curiously, Rad54 also disrupts D loops. How these opposing activities are coordinated toward productive recombination is unknown. Moreover, a seemingly disparate function of Rad54 is removal of Rad51 from heteroduplex DNA (hDNA) to allow HR-associated DNA synthesis. Here, we uncover features of D loop formation/dissociation dynamics, employing Rad51 filaments formed on ssDNAs that mimic the physiological length and structure of in vivo substrates. The Rad54 motor is activated by Rad51 bound to synapsed DNAs and guided by a ssDNA-binding domain. We present a unified model wherein Rad54 acts as an hDNA pump that drives D loop formation while simultaneously removing Rad51 from hDNA, consolidating both ATP-dependent activities of Rad54 into a single mechanistic step.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

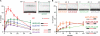
References
-
- Alexeev A, Mazin A, Kowalczykowski SC. Rad54 protein possesses chromatin-remodeling activity stimulated by a Rad51-ssDNA nucleoprotein filament. Nature Struct Biol. 2003;10:182–186. - PubMed
-
- Alexiadis V, Lusser A, Kadonaga JT. A conserved N-terminal motif in Rad54 is important for chromatin remodeling and homologous strand pairing. J Biol Chem. 2004;279:27824–27829. - PubMed
-
- Amitani I, Baskin RJ, Kowalczykowski SC. Visualization of Rad54, a chromatin remodeling protein, translocating on single DNA molecules. Mol Cell. 2006;23:143–148. - PubMed
-
- Bianchi M, DasGupta C, Radding CM. Synapsis and the formation of paranemic joints by E. coli RecA protein. Cell. 1983;34:931–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

