Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids
- PMID: 24486069
- PMCID: PMC4112383
- DOI: 10.1016/j.jaci.2013.12.1042
Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids
Abstract
Background: To date, genome-wide association studies (GWASs) of inhaled corticosteroid (ICS) response in asthmatic patients have focused primarily on lung function and exacerbations.
Objective: We hypothesized that GWAS analysis could identify novel genetic markers predicting a symptomatic response to ICSs.
Methods: We analyzed differences in asthma symptoms in response to ICSs in 124 white children from the Childhood Asthma Management Program (CAMP) trial using scores from diary cards. Of the 440,862 single nucleotide polymorphisms (SNPs) analyzed, the top 100 ranked SNPs were pursued for replication initially in subjects from the pediatric Childhood Asthma Research and Education trials (77 white children) and then in subjects from the adult Asthma Clinical Research Network (110 white adults) and Leukotriene Modifier or Corticosteroid or Corticosteroid-Salmeterol trials (110 white adults).
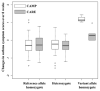
Results: The lowest P value for GWAS analysis in the CAMP trial was 8.94 × 10(-8) (rs2388639). Of the 60 SNPs available in the Childhood Asthma Research and Education Network trials, rs1558726 (combined P = 1.02 × 10(-5)), rs2388639 (combined P = 8.56 × 10(-9)), and rs10044254 (combined P = 9.16 × 10(-8)) independently replicated. However, these 3 SNPs were not additionally replicated in the adult asthmatic patients of the remaining trials. rs10044254 lies in the intronic region of F-box and leucine-rich repeat protein 7 (FBXL7) and is associated with decreased expression in immortalized B cells derived from CAMP participants.
Conclusions: We have identified a novel SNP, rs10044254, associated with both decreased expression of FBXL7 and improved symptomatic response to ICSs in 2 independent pediatric cohorts. Our results suggest that there might be a specific genetic mechanism regulating symptomatic response to ICSs in children that does not carry over to adults.
Keywords: Asthma; child; glucocorticoid; pharmacogenomics; polymorphism.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
Figures

References
-
- The Global Initiative for Asthma (GINA) The guideline for asthma management and prevention. [Accessed January 28, 2013];2010 Available at: http://www.ginasthma.org/guidelines-pocket-guide-for-asthma-management.html.
-
- The National Asthma Education and Prevention Program (NAEPP) National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. [Accessed January 28, 2013];2007 Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
-
- British Thoracic Society, Scottish Intercollegiateh Guidelines Network. British guideline on the management of asthma. [Accessed January 28, 2013]; Available at: http://www.sign.ac.uk/pdf/sign101.pdf.
-
- Boushey HA. Effects of inhaled corticosteroids on the consequences of asthma. J Allergy Clin Immunol. 1998;102(Suppl):S5–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

