Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus
- PMID: 24486087
- PMCID: PMC3988472
- DOI: 10.1016/j.neuron.2013.12.033
Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus
Abstract
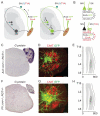
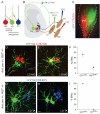
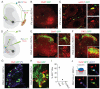
Primary sensory neurons convey information from the external world to relay circuits within the CNS, but the identity and organization of the neurons that process incoming sensory information remains sketchy. Within the CNS, viral tracing techniques that rely on retrograde transsynaptic transfer provide a powerful tool for delineating circuit organization. Viral tracing of the circuits engaged by primary sensory neurons has, however, been hampered by the absence of a genetically tractable anterograde transfer system. In this study, we demonstrate that rabies virus can infect sensory neurons in the somatosensory system, is subject to anterograde transsynaptic transfer from primary sensory to spinal target neurons, and can delineate output connectivity with third-order neurons. Anterograde transsynaptic transfer is a feature shared by other classes of primary sensory neurons, permitting the identification and potentially the manipulation of neural circuits processing sensory feedback within the mammalian CNS.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Vesicular stomatitis virus with the rabies virus glycoprotein directs retrograde transsynaptic transport among neurons in vivo.Front Neural Circuits. 2013 Feb 7;7:11. doi: 10.3389/fncir.2013.00011. eCollection 2013. Front Neural Circuits. 2013. PMID: 23403489 Free PMC article.
-
Synaptic Specificity and Application of Anterograde Transsynaptic AAV for Probing Neural Circuitry.J Neurosci. 2020 Apr 15;40(16):3250-3267. doi: 10.1523/JNEUROSCI.2158-19.2020. Epub 2020 Mar 20. J Neurosci. 2020. PMID: 32198185 Free PMC article.
-
Identification of Two Classes of Somatosensory Neurons That Display Resistance to Retrograde Infection by Rabies Virus.J Neurosci. 2017 Oct 25;37(43):10358-10371. doi: 10.1523/JNEUROSCI.1277-17.2017. Epub 2017 Sep 26. J Neurosci. 2017. PMID: 28951448 Free PMC article.
-
Rabies as a transneuronal tracer of circuits in the central nervous system.J Neurosci Methods. 2000 Nov 15;103(1):63-71. doi: 10.1016/s0165-0270(00)00296-x. J Neurosci Methods. 2000. PMID: 11074096 Review.
-
Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis.Dev Biol (Basel). 2008;131:493-506. Dev Biol (Basel). 2008. PMID: 18634512 Review.
Cited by
-
Fast, high-throughput production of improved rabies viral vectors for specific, efficient and versatile transsynaptic retrograde labeling.Elife. 2022 Aug 30;11:e79848. doi: 10.7554/eLife.79848. Elife. 2022. PMID: 36040301 Free PMC article.
-
Spinal V1 inhibitory interneuron clades differ in birthdate, projections to motoneurons, and heterogeneity.Elife. 2024 Nov 28;13:RP95172. doi: 10.7554/eLife.95172. Elife. 2024. PMID: 39607843 Free PMC article.
-
Proximal and distal spinal neurons innervating multiple synergist and antagonist motor pools.Elife. 2021 Nov 2;10:e70858. doi: 10.7554/eLife.70858. Elife. 2021. PMID: 34727018 Free PMC article.
-
A method to investigate muscle target-specific transcriptional signatures of single motor neurons.Dev Dyn. 2023 Jan;252(1):208-219. doi: 10.1002/dvdy.507. Epub 2022 Jul 8. Dev Dyn. 2023. PMID: 35705847 Free PMC article.
-
Rabies Virus CVS-N2c(ΔG) Strain Enhances Retrograde Synaptic Transfer and Neuronal Viability.Neuron. 2016 Feb 17;89(4):711-24. doi: 10.1016/j.neuron.2016.01.004. Epub 2016 Jan 21. Neuron. 2016. PMID: 26804990 Free PMC article.
References
-
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. - PubMed
-
- Astic L, Saucier D, Coulon P, Lafay F, Flamand A. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res. 1993;619:146–156. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

