Innate immunity and cardiomyocytes in ischemic heart disease
- PMID: 24486305
- PMCID: PMC3970925
- DOI: 10.1016/j.lfs.2014.01.062
Innate immunity and cardiomyocytes in ischemic heart disease
Abstract
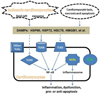
Myocardial ischemia/reperfusion (I/R) is the most common cause of myocardial inflammation, which is primarily a manifestation of the innate immune responses. Innate immunity is activated when pattern recognition receptors (PRRs) respond to molecular patterns common to microbes and to danger signals expressed by injured or infected cells, so called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). The expression of various PRRs in cardiomyocytes and the release of DAMPs from cardiomyocytes subjected to I/R injury, through active mechanisms as well as passive processes, enable cardiomyocytes to generate innate immune responses. Studies in isolated heart and cardiomyocytes have confirmed the inflammatory and functional effects of cardiac PRRs especially Toll-like receptors in response to I/R-derived DAMPs, such as heat shock proteins. This review addresses the active role of cardiomyocytes in mediating innate inflammatory responses to myocardial I/R. We propose that cardiomyocytes act as innate immune cells in myocardial I/R injury.
Keywords: Cardiomyocytes; Heart; Inflammation; Innate immunity; Ischemia/reperfusion; NF-κB; Pattern recognition receptor; TLR2; TLR4.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest: None declared.
Figures
Similar articles
-
Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success.Front Immunol. 2020 Dec 8;11:599511. doi: 10.3389/fimmu.2020.599511. eCollection 2020. Front Immunol. 2020. PMID: 33363540 Free PMC article. Review.
-
Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion.Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2805-13. doi: 10.1152/ajpheart.00299.2008. Epub 2008 Apr 25. Am J Physiol Heart Circ Physiol. 2008. PMID: 18441202 Free PMC article.
-
Bridging innate immunity and myocardial ischemia/reperfusion injury: the search for therapeutic targets.Curr Pharm Des. 2008;14(12):1205-16. doi: 10.2174/138161208784246090. Curr Pharm Des. 2008. PMID: 18473868 Review.
-
Paying for the Tolls: The High Cost of the Innate Immune System for the Cardiac Myocyte.Adv Exp Med Biol. 2017;1003:17-34. doi: 10.1007/978-3-319-57613-8_2. Adv Exp Med Biol. 2017. PMID: 28667552 Review.
-
The innate immune response in reperfused myocardium.Cardiovasc Res. 2012 May 1;94(2):276-83. doi: 10.1093/cvr/cvs018. Epub 2012 Jan 20. Cardiovasc Res. 2012. PMID: 22266751 Review.
Cited by
-
Can the calcium-regulating hormones counteract the detrimental impact of pro-inflammatory damage-associated molecular patterns in the development of heart failure?J Investig Med. 2021 Aug;69(6):1148-1152. doi: 10.1136/jim-2020-001754. Epub 2021 May 5. J Investig Med. 2021. PMID: 33952612 Free PMC article. Review.
-
FOXC1 up-regulates the expression of toll-like receptors in myocardial ischaemia.J Cell Mol Med. 2019 Nov;23(11):7566-7580. doi: 10.1111/jcmm.14626. Epub 2019 Sep 13. J Cell Mol Med. 2019. PMID: 31517441 Free PMC article.
-
TLR4 Inhibition Attenuated LPS-Induced Proinflammatory Signaling and Cytokine Release in Mouse Hearts and Cardiomyocytes.Immun Inflamm Dis. 2025 Jan;13(1):e70133. doi: 10.1002/iid3.70133. Immun Inflamm Dis. 2025. PMID: 39853914 Free PMC article.
-
Vagal modulation of high mobility group box-1 protein mediates electroacupuncture-induced cardioprotection in ischemia-reperfusion injury.Sci Rep. 2015 Oct 26;5:15503. doi: 10.1038/srep15503. Sci Rep. 2015. PMID: 26499847 Free PMC article.
-
Pro-inflammatory cytokines as emerging molecular determinants in cardiolaminopathies.J Cell Mol Med. 2021 Dec;25(23):10902-10915. doi: 10.1111/jcmm.16975. Epub 2021 Nov 12. J Cell Mol Med. 2021. PMID: 34773379 Free PMC article.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. - PubMed
-
- Anderson KV, Bokla L, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42(3):791–798. - PubMed
-
- Anderson KV, Jürgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42(3):779–789. - PubMed
-
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbäurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

