Induced overexpression of phospholemman S68E mutant improves cardiac contractility and mortality after ischemia-reperfusion
- PMID: 24486513
- PMCID: PMC3962630
- DOI: 10.1152/ajpheart.00861.2013
Induced overexpression of phospholemman S68E mutant improves cardiac contractility and mortality after ischemia-reperfusion
Abstract
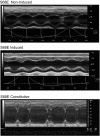
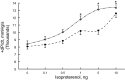
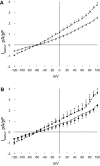
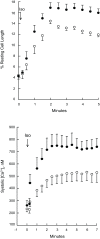
Phospholemman (PLM), when phosphorylated at Ser(68), inhibits cardiac Na+ / Ca2+ exchanger 1 (NCX1) and relieves its inhibition on Na+ -K+ -ATPase. We have engineered mice in which expression of the phosphomimetic PLM S68E mutant was induced when dietary doxycycline was removed at 5 wk. At 8-10 wk, compared with noninduced or wild-type hearts, S68E expression in induced hearts was ∼35-75% that of endogenous PLM, but protein levels of sarco(endo)plasmic reticulum Ca2+ -ATPase, α1- and α2-subunits of Na+ -K+ -ATPase, α1c-subunit of L-type Ca2+ channel, and phosphorylated ryanodine receptor were unchanged. The NCX1 protein level was increased by ∼47% but the NCX1 current was depressed by ∼34% in induced hearts. Isoproterenol had no effect on NCX1 currents but stimulated Na+ -K+ -ATPase currents equally in induced and noninduced myocytes. At baseline, systolic intracellular Ca2+ concentrations ([Ca2+]i), sarcoplasmic reticulum Ca2+ contents, and [Ca(2+)]i transient and contraction amplitudes were similar between induced and noninduced myocytes. Isoproterenol stimulation resulted in much higher systolic [Ca2+]i, sarcoplasmic reticulum Ca2+ content, and [Ca2+]i transient and contraction amplitudes in induced myocytes. Echocardiography and in vivo close-chest catheterization demonstrated similar baseline myocardial function, but isoproterenol induced a significantly higher +dP/dt in induced compared with noninduced hearts. In contrast to the 50% mortality observed in mice constitutively overexpressing the S68E mutant, induced mice had similar survival as wild-type and noninduced mice. After ischemia-reperfusion, despite similar areas at risk and left ventricular infarct sizes, induced mice had significantly higher +dP/dt and -dP/dt and lower perioperative mortality compared with noninduced mice. We propose that phosphorylated PLM may be a novel therapeutic target in ischemic heart disease.
Keywords: FXYD proteins; in vivo hemodynamics; intracellular Ca2+ regulation; ischemic cardiomyopathy.
Figures








Similar articles
-
Regulation of in vivo cardiac contractility by phospholemman: role of Na+/Ca2+ exchange.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H859-68. doi: 10.1152/ajpheart.00894.2010. Epub 2010 Dec 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21193587 Free PMC article.
-
Constitutive overexpression of phosphomimetic phospholemman S68E mutant results in arrhythmias, early mortality, and heart failure: potential involvement of Na+/Ca2+ exchanger.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H770-81. doi: 10.1152/ajpheart.00733.2011. Epub 2011 Nov 11. Am J Physiol Heart Circ Physiol. 2012. PMID: 22081699 Free PMC article.
-
Phospholemman and beta-adrenergic stimulation in the heart.Am J Physiol Heart Circ Physiol. 2010 Mar;298(3):H807-15. doi: 10.1152/ajpheart.00877.2009. Epub 2009 Dec 11. Am J Physiol Heart Circ Physiol. 2010. PMID: 20008271 Free PMC article.
-
Coordinated regulation of cardiac Na(+)/Ca (2+) exchanger and Na (+)-K (+)-ATPase by phospholemman (FXYD1).Adv Exp Med Biol. 2013;961:175-90. doi: 10.1007/978-1-4614-4756-6_15. Adv Exp Med Biol. 2013. PMID: 23224879 Review.
-
Regulation of cardiac Na+/Ca2+ exchanger by phospholemman.Ann N Y Acad Sci. 2007 Mar;1099:119-34. doi: 10.1196/annals.1387.004. Ann N Y Acad Sci. 2007. PMID: 17446450 Review.
Cited by
-
Design of a Proteolytically Stable Sodium-Calcium Exchanger 1 Activator Peptide for In Vivo Studies.Front Pharmacol. 2021 Jun 7;12:638646. doi: 10.3389/fphar.2021.638646. eCollection 2021. Front Pharmacol. 2021. PMID: 34163352 Free PMC article.
-
FXYD proteins and sodium pump regulatory mechanisms.J Gen Physiol. 2021 Apr 5;153(4):e202012633. doi: 10.1085/jgp.202012633. J Gen Physiol. 2021. PMID: 33688925 Free PMC article. Review.
-
Ca²⁺ entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance.Am J Physiol Heart Circ Physiol. 2015 Mar 15;308(6):H637-50. doi: 10.1152/ajpheart.00720.2014. Epub 2015 Jan 9. Am J Physiol Heart Circ Physiol. 2015. PMID: 25576627 Free PMC article.
-
BAG3 regulates contractility and Ca(2+) homeostasis in adult mouse ventricular myocytes.J Mol Cell Cardiol. 2016 Mar;92:10-20. doi: 10.1016/j.yjmcc.2016.01.015. Epub 2016 Jan 19. J Mol Cell Cardiol. 2016. PMID: 26796036 Free PMC article.
-
Data-Independent Acquisition Proteomics and N-Terminomics Methods Reveal Alterations in Mitochondrial Function and Metabolism in Ischemic-Reperfused Hearts.J Proteome Res. 2024 Feb 2;23(2):844-856. doi: 10.1021/acs.jproteome.3c00754. Epub 2024 Jan 24. J Proteome Res. 2024. PMID: 38264990 Free PMC article.
References
-
- Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882, 2005 - PubMed
-
- Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292: H2119–H2130, 2007 - PubMed
-
- Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterisation of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na/K ATPase activity. Am J Physiol Heart Circ Physiol 294: H613–H621, 2008 - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

