Degradation of connexins and gap junctions
- PMID: 24486527
- PMCID: PMC3989500
- DOI: 10.1016/j.febslet.2014.01.031
Degradation of connexins and gap junctions
Abstract
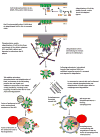
Connexin proteins are short-lived within the cell, whether present in the secretory pathway or in gap junction plaques. Their levels can be modulated by their rate of degradation. Connexins, at different stages of assembly, are degraded through the proteasomal, endo-/lysosomal, and phago-/lysosomal pathways. In this review, we summarize the current knowledge about connexin and gap junction degradation including the signals and protein-protein interactions that participate in their targeting for degradation.
Keywords: Autophagosome; Connexin; Gap junction; Lysosome; Proteasome; Ubiquitin.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–35. - PubMed
-
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62:256–67. - PubMed
-
- Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

