Role of Bcl-xL/Beclin-1 in interplay between apoptosis and autophagy in oxaliplatin and bortezomib-induced cell death
- PMID: 24486574
- PMCID: PMC3969586
- DOI: 10.1016/j.bcp.2014.01.027
Role of Bcl-xL/Beclin-1 in interplay between apoptosis and autophagy in oxaliplatin and bortezomib-induced cell death
Abstract
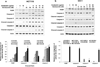
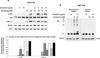
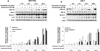
Recent studies indicate that a complex relationship exists between autophagy and apoptosis. In this study we investigated a regulatory relationship between autophagy and apoptosis in colorectal cancer cells utilizing molecular and biochemical approaches. For this study, human colorectal carcinoma HCT116 and CX-1 cells were treated with two chemotherapeutic agents-oxaliplatin, which induces apoptosis, and bortezomib, which triggers both apoptosis and autophagy. A combinatorial treatment of oxaliplatin and bortezomib caused a synergistic induction of apoptosis which was mediated through an increase in caspase activation. The combinational treatment of oxaliplatin and bortezomib promoted the JNK-Bcl-xL-Bax pathway which modulated the synergistic effect through the mitochondria-dependent apoptotic pathway. JNK signaling led to Bcl-xL phosphorylation at serine 62, oligomerization of Bax, alteration of mitochondrial membrane potential, and subsequent cytochrome c release. Overexpression of dominant-negative mutant of Bcl-xL (S62A), but not dominant-positive mutant of Bcl-xL (S62D), suppressed cytochrome c release and synergistic death effect. Interestingly, Bcl-xL also affected autophagy through alteration of interaction with Beclin-1. Beclin-1 was dissociated from Bcl-xL and initiated autophagy during treatment with oxaliplatin and bortezomib. However, activated caspase 8 cleaved Beclin-1 and suppressed Beclin-1-associated autophagy and enhanced apoptosis. A combinatorial treatment of oxaliplatin and bortezomib-induced Beclin-1 cleavage was abolished in Beclin-1 double mutant (D133AA/D149A) knock-in HCT116 cells, restoring the autophagy-promoting function of Beclin-1 and suppressing the apoptosis induced by the combination therapy. In addition, the combinatorial treatment significantly inhibited colorectal cancer xenografts' tumor growth. An understanding of the molecular mechanisms of crosstalk between apoptosis and autophagy will support the application of combinatorial treatment to colorectal cancer.
Keywords: Bcl-xL; Beclin-1; Bortezomib; Mitochondria-dependent pathway; Oxaliplatin.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures







References
-
- Cancer Facts & Figures. American Cancer Society; 2013.
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. - PubMed
-
- Rothenberg ML, Meropol NJ, Poplin EA, Van Cutsem E, Wadler S. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol. 2001;18:3801–3807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

