Cross-talk between EphA2 and BRaf/CRaf is a key determinant of response to Dasatinib
- PMID: 24486585
- PMCID: PMC3975695
- DOI: 10.1158/1078-0432.CCR-13-2141
Cross-talk between EphA2 and BRaf/CRaf is a key determinant of response to Dasatinib
Abstract
Purpose: EphA2 is an attractive therapeutic target because of its diverse roles in cancer growth and progression. Dasatinib is a multikinase inhibitor that targets EphA2 and other kinases. However, reliable predictive markers and a better understanding of the mechanisms of response to this agent are needed.
Experimental design: The effects of dasatinib on human uterine cancer cell lines were examined using a series of in vitro experiments, including MTT, Western blot analysis, and plasmid transfection. In vivo, an orthotopic mouse model of uterine cancer was utilized to identify the biologic effects of dasatinib. Molecular markers for response prediction and the mechanisms relevant to response to dasatinib were identified by using reverse phase protein array (RPPA), immunoprecipitation, and double immunofluorescence staining.
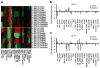
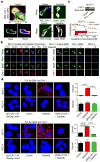
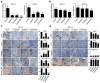
Results: We show that high levels of CAV-1, EphA2 phosphorylation at S897, and the status of PTEN are key determinants of dasatinib response in uterine carcinoma. A set of markers essential for dasatinib response was also identified and includes CRaf, pCRaf(S338), pMAPK(T202/Y204) (mitogen-activated protein kinase [MAPK] pathway), pS6(S240/244), p70S6k(T389) (mTOR pathway), and pAKT(S473). A novel mechanism for response was discovered whereby high expression level of CAV-1 at the plasma membrane disrupts the BRaf/CRaf heterodimer and thus inhibits the activation of MAPK pathway during dasatinib treatment.
Conclusions: Our in vitro and in vivo results provide a new understanding of EphA2 targeting by dasatinib and identify key predictors of therapeutic response. These findings have implications for ongoing dasatinib-based clinical trials.
©2014 AACR.
Conflict of interest statement
Figures


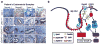
Similar articles
-
MEK inhibition overcomes resistance to EphA2-targeted therapy in uterine cancer.Gynecol Oncol. 2021 Oct;163(1):181-190. doi: 10.1016/j.ygyno.2021.08.003. Epub 2021 Aug 11. Gynecol Oncol. 2021. PMID: 34391578 Free PMC article.
-
Activity of the multikinase inhibitor dasatinib against ovarian cancer cells.Br J Cancer. 2009 Nov 17;101(10):1699-708. doi: 10.1038/sj.bjc.6605381. Epub 2009 Oct 27. Br J Cancer. 2009. PMID: 19861960 Free PMC article.
-
Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer.Br J Cancer. 2008 Oct 7;99(7):1074-82. doi: 10.1038/sj.bjc.6604676. Epub 2008 Sep 16. Br J Cancer. 2008. PMID: 18797457 Free PMC article.
-
Raf kinases in cancer-roles and therapeutic opportunities.Oncogene. 2011 Aug 11;30(32):3477-88. doi: 10.1038/onc.2011.160. Epub 2011 May 16. Oncogene. 2011. PMID: 21577205 Review.
-
Structural snapshots of RAF kinase interactions.Biochem Soc Trans. 2018 Dec 17;46(6):1393-1406. doi: 10.1042/BST20170528. Epub 2018 Oct 31. Biochem Soc Trans. 2018. PMID: 30381334 Review.
Cited by
-
Targeting Host Tyrosine Kinase Receptor EPHA2 Signaling Affects Uropathogen Infection in Human Bladder Epithelial Cells.Pathogens. 2022 Oct 12;11(10):1176. doi: 10.3390/pathogens11101176. Pathogens. 2022. PMID: 36297233 Free PMC article.
-
Eph receptors and ephrins: therapeutic opportunities.Annu Rev Pharmacol Toxicol. 2015;55:465-87. doi: 10.1146/annurev-pharmtox-011112-140226. Epub 2014 Oct 3. Annu Rev Pharmacol Toxicol. 2015. PMID: 25292427 Free PMC article. Review.
-
Cytomegalovirus US28 regulates cellular EphA2 to maintain viral latency.Sci Adv. 2022 Oct 28;8(43):eadd1168. doi: 10.1126/sciadv.add1168. Epub 2022 Oct 26. Sci Adv. 2022. PMID: 36288299 Free PMC article.
-
Decreased expression of receptor tyrosine kinase of EphB1 protein in renal cell carcinomas.Int J Clin Exp Pathol. 2014 Jun 15;7(7):4254-60. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25120806 Free PMC article.
-
Unveiling the therapeutic promise of EphA2 in glioblastoma: a comprehensive review.Discov Oncol. 2024 Sep 27;15(1):501. doi: 10.1007/s12672-024-01380-8. Discov Oncol. 2024. PMID: 39331302 Free PMC article. Review.
References
-
- Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–87. - PubMed
-
- Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech. 2002;59:58–67. - PubMed
-
- Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–40. - PubMed
-
- Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. - PubMed
-
- Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

