Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors
- PMID: 24486636
- PMCID: PMC3992482
- DOI: 10.1016/j.cct.2014.01.010
Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors
Abstract
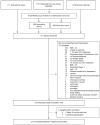
Obesity is a risk factor for breast cancer recurrence and death. Women who reside in rural areas have higher obesity prevalence and suffer from breast cancer treatment-related disparities compared to urban women. The objective of this 5-year randomized controlled trial is to compare methods for delivering extended care for weight loss maintenance among rural breast cancer survivors. Group phone-based counseling via conference calls addresses access barriers, is more cost-effective than individual phone counseling, and provides group support which may be ideal for rural breast cancer survivors who are more likely to have unmet support needs. Women (n=210) diagnosed with Stage 0 to III breast cancer in the past 10 years who are ≥ 3 months out from initial cancer treatments, have a BMI 27-45 kg/m(2), and have physician clearance were enrolled from multiple cancer centers. During Phase I (months 0 to 6), all women receive a behavioral weight loss intervention delivered through group phone sessions. Women who successfully lose 5% of weight enter Phase II (months 6 to 18) and are randomized to one of two extended care arms: continued group phone-based treatment or a mail-based newsletter. During Phase III, no contact is made (months 18 to 24). The primary outcome is weight loss maintenance from 6 to 18 months. Secondary outcomes include quality of life, serum biomarkers, and cost-effectiveness. This study will provide essential information on how to reach rural survivors in future efforts to establish weight loss support for breast cancer survivors as a standard of care.
Trial registration: ClinicalTrials.gov NCT01441011.
Keywords: Behavioral weight control; Breast cancer; Obesity; Quality of life; Rural.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
-
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–43. - PubMed
-
- Cleveland RJ, Eng SM, Abrahamson PE, Britton JA, Teitelbaum SL, Neugut AI, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1803–11. - PubMed
-
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. - PubMed
-
- Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–42. - PubMed
-
- Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–9. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous