Population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children with tuberculosis: in silico evaluation of currently recommended doses
- PMID: 24486870
- PMCID: PMC3977610
- DOI: 10.1093/jac/dkt524
Population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children with tuberculosis: in silico evaluation of currently recommended doses
Abstract
Objectives: To describe the population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children and evaluate the adequacy of steady-state exposures.
Patients and methods: We used previously published data for 76 South African children with tuberculosis to describe the population pharmacokinetics of rifampicin, pyrazinamide and isoniazid. Monte Carlo simulations were used to predict steady-state exposures in children following doses in fixed-dose combination tablets in accordance with the revised guidelines. Reference exposures were derived from an ethnically similar adult population with tuberculosis taking currently recommended doses.
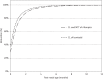
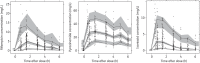
Results: The final models included allometric scaling of clearance and volume of distribution using body weight. Maturation was included for clearance of isoniazid and clearance and absorption transit time of rifampicin. For a 2-year-old child weighing 12.5 kg, the estimated typical oral clearances of rifampicin and pyrazinamide were 8.15 and 1.08 L/h, respectively. Isoniazid typical oral clearance (adjusted for bioavailability) was predicted to be 4.44, 11.6 and 14.6 L/h for slow, intermediate and fast acetylators, respectively. Higher oral clearance values in intermediate and fast acetylators also resulted from 23% lower bioavailability compared with slow acetylators.
Conclusions: Simulations based on our models suggest that with the new WHO dosing guidelines and utilizing available paediatric fixed-dose combinations, children will receive adequate rifampicin exposures when compared with adults, but with a larger degree of variability. However, pyrazinamide and isoniazid exposures in many children will be lower than in adults. Further studies are needed to confirm these findings in children administered the revised dosages and to optimize pragmatic approaches to dosing.
Keywords: NONMEM; anti-Mycobacterium; modelling and simulation; paediatrics; pharmacometrics.
Figures

Similar articles
-
Pharmacokinetics of anti-tuberculosis drugs in Venezuelan children younger than 16 years of age: supportive evidence for the implementation of revised WHO dosing recommendations.Trop Med Int Health. 2012 Dec;17(12):1449-56. doi: 10.1111/tmi.12003. Epub 2012 Oct 24. Trop Med Int Health. 2012. PMID: 23094704
-
Optimizing Dosing and Fixed-Dose Combinations of Rifampicin, Isoniazid, and Pyrazinamide in Pediatric Patients With Tuberculosis: A Prospective Population Pharmacokinetic Study.Clin Infect Dis. 2022 Aug 24;75(1):141-151. doi: 10.1093/cid/ciab908. Clin Infect Dis. 2022. PMID: 34665866 Free PMC article.
-
Evaluating pediatric tuberculosis dosing guidelines: A model-based individual data pooled analysis.PLoS Med. 2023 Nov 21;20(11):e1004303. doi: 10.1371/journal.pmed.1004303. eCollection 2023 Nov. PLoS Med. 2023. PMID: 37988391 Free PMC article.
-
Pharmacokinetics of First-Line Anti-Tubercular Drugs.Indian J Pediatr. 2019 May;86(5):468-478. doi: 10.1007/s12098-019-02911-w. Epub 2019 Mar 26. Indian J Pediatr. 2019. PMID: 30915644 Review.
-
[Pharmacokinetic aspects of tuberculosis therapy with a fixed combination of rifampicin, isoniazide and pyrazinamide].Z Gesamte Inn Med. 1991 Jun;46(8):276-9. Z Gesamte Inn Med. 1991. PMID: 1897286 Review. German.
Cited by
-
Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis.Clin Infect Dis. 2016 Oct 1;63(7):e147-e195. doi: 10.1093/cid/ciw376. Epub 2016 Aug 10. Clin Infect Dis. 2016. PMID: 27516382 Free PMC article.
-
Determination of Rifampin Concentrations by Urine Colorimetry and Mobile Phone Readout for Personalized Dosing in Tuberculosis Treatment.J Pediatric Infect Dis Soc. 2021 Mar 26;10(2):104-111. doi: 10.1093/jpids/piaa024. J Pediatric Infect Dis Soc. 2021. PMID: 32170944 Free PMC article.
-
Parametric Population Pharmacokinetics Model Repository of Rifampicin: Model-Informed Individualized Therapy.Clin Pharmacol. 2025 Apr 15;17:49-78. doi: 10.2147/CPAA.S502272. eCollection 2025. Clin Pharmacol. 2025. PMID: 40255481 Free PMC article.
-
Population Pharmacokinetic Analysis of Rifampicin in Plasma, Cerebrospinal Fluid, and Brain Extracellular Fluid in South African Children with Tuberculous Meningitis.Antimicrob Agents Chemother. 2023 Mar 16;67(3):e0147422. doi: 10.1128/aac.01474-22. Epub 2023 Feb 23. Antimicrob Agents Chemother. 2023. PMID: 36815838 Free PMC article.
-
Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in Indian children.BMC Infect Dis. 2015 Mar 14;15:126. doi: 10.1186/s12879-015-0862-7. BMC Infect Dis. 2015. PMID: 25887748 Free PMC article.
References
-
- WHO. Global Tuberculosis Report 2013. Geneva: WHO; http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. (31 December 2013, date last accessed)
-
- Marais BJ, Hesseling AC, Gie RP, et al. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–63. - PubMed
-
- Hu Y, Coates AR, Mitchison DA. Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2006;10:317–22. - PubMed
-
- Steele MA, Des Prez RM. The role of pyrazinamide in tuberculosis chemotherapy. Chest. 1988;94:845–50. - PubMed
-
- Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4:796–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

