Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment
- PMID: 24486952
- PMCID: PMC3975078
- DOI: 10.1016/j.antiviral.2014.01.016
Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment
Abstract
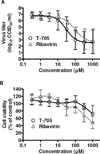
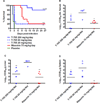
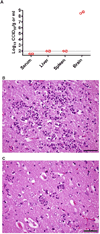
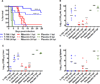
Rift Valley fever is a zoonotic, arthropod-borne disease that affects livestock and humans. The etiologic agent, Rift Valley fever virus (RVFV; Bunyaviridae, Phlebovirus) is primarily transmitted through mosquito bites, but can also be transmitted by exposure to infectious aerosols. There are presently no licensed vaccines or therapeutics to prevent or treat severe RVFV infection in humans. We have previously reported on the activity of favipiravir (T-705) against the MP-12 vaccine strain of RVFV and other bunyaviruses in cell culture. In addition, efficacy has also been documented in mouse and hamster models of infection with the related Punta Toro virus. Here, hamsters challenged with the highly pathogenic ZH501 strain of RVFV were used to evaluate the activity of favipiravir against lethal infection. Subcutaneous RVFV challenge resulted in substantial serum and tissue viral loads and caused severe disease and mortality within 2-3 days of infection. Oral favipiravir (200 mg/kg/day) prevented mortality in 60% or greater of hamsters challenged with RVFV when administered within 1 or 6h post-exposure and reduced RVFV titers in serum and tissues relative to the time of treatment initiation. In contrast, although ribavirin (75 mg/kg/day) was effective at protecting animals from the peracute RVFV disease, most ultimately succumbed from a delayed-onset neurologic disease associated with high RVFV burden observed in the brain in moribund animals. When combined, T-705 and ribavirin treatment started 24 h post-infection significantly improved survival outcome and reduced serum and tissue virus titers compared to monotherapy. Our findings demonstrate significant post-RVFV exposure efficacy with favipiravir against both peracute disease and delayed-onset neuroinvasion, and suggest added benefit when combined with ribavirin.
Keywords: Bunyavirus; Favipiravir (T-705); Phlebovirus; Ribavirin; Rift Valley fever virus.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters.Antiviral Res. 2018 Aug;156:38-45. doi: 10.1016/j.antiviral.2018.05.013. Epub 2018 Jun 1. Antiviral Res. 2018. PMID: 29864447 Free PMC article.
-
Rift Valley fever virus infection in golden Syrian hamsters.PLoS One. 2015 Jan 21;10(1):e0116722. doi: 10.1371/journal.pone.0116722. eCollection 2015. PLoS One. 2015. PMID: 25607955 Free PMC article.
-
Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever.PLoS Negl Trop Dis. 2014 Apr 10;8(4):e2790. doi: 10.1371/journal.pntd.0002790. eCollection 2014 Apr. PLoS Negl Trop Dis. 2014. PMID: 24722586 Free PMC article.
-
Single-cycle replicable Rift Valley fever virus mutants as safe vaccine candidates.Virus Res. 2016 May 2;216:55-65. doi: 10.1016/j.virusres.2015.05.012. Epub 2015 May 27. Virus Res. 2016. PMID: 26022573 Free PMC article. Review.
-
Rift Valley Fever Virus Encephalitis: Viral and Host Determinants of Pathogenesis.Annu Rev Virol. 2024 Sep;11(1):309-325. doi: 10.1146/annurev-virology-093022-011544. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38635867 Review.
Cited by
-
Computer-Selected Antiviral Compounds: Assessing In Vitro Efficacies against Rift Valley Fever Virus.Viruses. 2024 Jan 5;16(1):88. doi: 10.3390/v16010088. Viruses. 2024. PMID: 38257788 Free PMC article.
-
Favipiravir (T-705) Protects IFNAR-/- Mice against Lethal Zika Virus Infection in a Sex-Dependent Manner.Microorganisms. 2021 May 29;9(6):1178. doi: 10.3390/microorganisms9061178. Microorganisms. 2021. PMID: 34072604 Free PMC article.
-
Clinical Evaluation of Ebola Virus Disease Therapeutics.Trends Mol Med. 2017 Sep;23(9):820-830. doi: 10.1016/j.molmed.2017.07.002. Epub 2017 Aug 17. Trends Mol Med. 2017. PMID: 28822631 Free PMC article. Review.
-
Rift Valley Fever Virus Infection Causes Acute Encephalitis in the Ferret.mSphere. 2020 Oct 28;5(5):e00798-20. doi: 10.1128/mSphere.00798-20. mSphere. 2020. PMID: 33115835 Free PMC article.
-
Structural and functional similarities in bunyaviruses: Perspectives for pan-bunya antivirals.Rev Med Virol. 2019 May;29(3):e2039. doi: 10.1002/rmv.2039. Epub 2019 Feb 11. Rev Med Virol. 2019. PMID: 30746831 Free PMC article. Review.
References
-
- Anderson GW, Jr, Slayter MV, Hall W, Peters CJ. Pathogenesis of a phleboviral infection (Punta Toro virus) in golden Syrian hamsters. Arch. Virol. 1990;114:203–212. - PubMed
-
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. - PubMed
-
- Ergonul O. Treatment of Crimean-Congo hemorrhagic fever. Antiviral Res. 2008 - PubMed
-
- Fisher AF, Tesh RB, Tonry J, Guzman H, Liu D, Xiao SY. Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus. Am. J. Trop. Med. Hyg. 2003;69:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

