MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury
- PMID: 24487387
- PMCID: PMC3962628
- DOI: 10.1152/ajplung.00272.2013
MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury
Abstract
Sepsis is a systemic inflammatory response to infection and a major cause of death worldwide. Because specific therapies to treat sepsis are limited, and underlying pathogenesis is unclear, current medical care remains purely supportive. Therefore targeted therapies to treat sepsis need to be developed. Although an important mediator of sepsis is thought to be mitochondrial dysfunction, the underlying molecular mechanism is unclear. Modulation of mitochondrial processes may be an effective therapeutic strategy in sepsis. Here, we investigated the role of the kinase MKK3 in regulation of mitochondrial function in sepsis. Using clinically relevant animal models, we examined mitochondrial function in primary mouse lung endothelial cells exposed to LPS. MKK3 deficiency reduces lethality of sepsis in mice and by lowering levels of lung and mitochondrial injury as well as reactive oxygen species. Furthermore, MKK3 deficiency appeared to simultaneously increase mitochondrial biogenesis and mitophagy through the actions of Sirt1, Pink1, and Parkin. This led to a more robust mitochondrial network, which we propose provides protection against sepsis. We also detected higher MKK3 activation in isolated peripheral blood mononuclear cells from septic patients compared with nonseptic controls. Our findings demonstrate a critical role for mitochondria in the pathogenesis of sepsis that involves a previously unrecognized function of MKK3 in mitochondrial quality control. This mitochondrial pathway may help reveal new diagnostic markers and therapeutic targets against sepsis.
Keywords: biogenesis; lung injury; mitochondria; mitogen-activated protein kinases; mitophagy; sepsis.
Figures

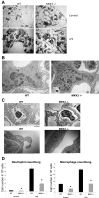

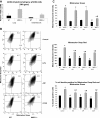



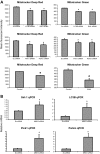

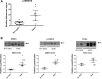
References
-
- Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004
-
- Adrie C, Bachelet M, Vayssier-Taussat M, Russo-Marie F, Bouchaert I, Adib-Conquy M, Cavaillon JM, Pinsky MR, Dhainaut JF, Polla BS. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med 164: 389–395, 2001 - PubMed
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ; ACCP/SCCM Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest 136: e28, 2009 - PubMed
-
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

