Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model
- PMID: 24487407
- PMCID: PMC3944496
- DOI: 10.1242/dmm.012922
Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model
Abstract
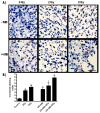
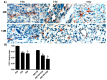
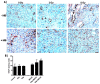
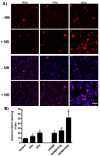
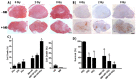
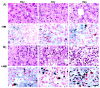
Tumor radiation resistance poses a major obstacle in achieving an optimal outcome in radiation therapy. In the current study, we characterize a novel therapeutic approach that combines ultrasound-driven microbubbles with radiation to increase treatment responses in a prostate cancer xenograft model in mice. Tumor response to ultrasound-driven microbubbles and radiation was assessed 24 hours after treatment, which consisted of radiation treatments alone (2 Gy or 8 Gy) or ultrasound-stimulated microbubbles only, or a combination of radiation and ultrasound-stimulated microbubbles. Immunohistochemical analysis using in situ end labeling (ISEL) and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) revealed increased cell death within tumors exposed to combined treatments compared with untreated tumors or tumors exposed to radiation alone. Several biomarkers were investigated to evaluate cell proliferation (Ki67), blood leakage (factor VIII), angiogenesis (cluster of differentiation molecule CD31), ceramide-formation, angiogenesis signaling [vascular endothelial growth factor (VEGF)], oxygen limitation (prolyl hydroxylase PHD2) and DNA damage/repair (γH2AX). Results demonstrated reduced vascularity due to vascular disruption by ultrasound-stimulated microbubbles, increased ceramide production and increased DNA damage of tumor cells, despite decreased tumor oxygenation with significantly less proliferating cells in the combined treatments. This combined approach could be a feasible option as a novel enhancing approach in radiation therapy.
Keywords: Angiogenesis; Microbubbles; Proliferation; Radiation; Ultrasound.
Figures



References
-
- Al-Mahrouki A. A., Karshafian R., Giles A., Czarnota G. J. (2012). Bioeffects of ultrasound-stimulated microbubbles on endothelial cells: gene expression changes associated with radiation enhancement in vitro. Ultrasound Med. Biol. 38, 1958–1969 - PubMed
-
- Badea R., Seicean A., Diaconu B., Stan-Iuga R., Sparchez Z., Tantau M., Socaciu M. (2009). Contrast-enhanced ultrasound of the pancreas – a method beyond its potential or a new diagnostic standard? J. Gastrointestin. Liver Dis. 18, 237–242 - PubMed
-
- Bao S., Wu Q., McLendon R. E., Hao Y., Shi Q., Hjelmeland A. B., Dewhirst M. W., Bigner D. D., Rich J. N. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 - PubMed
-
- Bastianutto C., Mian A., Symes J., Mocanu J., Alajez N., Sleep G., Shi W., Keating A., Crump M., Gospodarowicz M., et al. (2007). Local radiotherapy induces homing of hematopoietic stem cells to the irradiated bone marrow. Cancer Res. 67, 10112–10116 - PubMed
-
- Becher O. J., Hambardzumyan D., Walker T. R., Helmy K., Nazarian J., Albrecht S., Hiner R. L., Gall S., Huse J. T., Jabado N., et al. (2010). Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 70, 2548–2557 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

