Synaptic abnormalities in a Drosophila model of Alzheimer's disease
- PMID: 24487408
- PMCID: PMC3944497
- DOI: 10.1242/dmm.012104
Synaptic abnormalities in a Drosophila model of Alzheimer's disease
Abstract
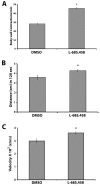
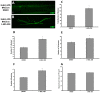
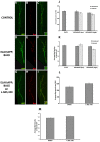
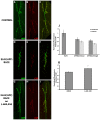
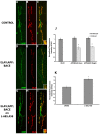
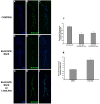
Alzheimer's disease (AD) is an age-related neurodegenerative disease characterized by memory loss and decreased synaptic function. Advances in transgenic animal models of AD have facilitated our understanding of this disorder, and have aided in the development, speed and efficiency of testing potential therapeutics. Recently, we have described the characterization of a novel model of AD in the fruit fly, Drosophila melanogaster, where we expressed the human AD-associated proteins APP and BACE in the central nervous system of the fly. Here we describe synaptic defects in the larval neuromuscular junction (NMJ) in this model. Our results indicate that expression of human APP and BACE at the larval NMJ leads to defective larval locomotion behavior, decreased presynaptic connections, altered mitochondrial localization in presynaptic motor neurons and decreased postsynaptic protein levels. Treating larvae expressing APP and BACE with the γ-secretase inhibitor L-685,458 suppresses the behavioral defects as well as the pre- and postsynaptic defects. We suggest that this model will be useful to assess and model the synaptic dysfunction normally associated with AD, and will also serve as a powerful in vivo tool for rapid testing of potential therapeutics for AD.
Keywords: APP; Alzheimer’s disease; BACE; Drosophila; NMJ; Synapse.
Figures



References
-
- Atwood H. L., Karunanithi S., Georgiou J., Charlton M. P. (1997). Strength of synaptic transmission at neuromuscular junctions of crustaceans and insects in relation to calcium entry. Invert. Neurosci. 3, 81–87 - PubMed
-
- Bonini N. M., Fortini M. E. (2003). Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci. 26, 627–656 - PubMed
-
- Braak H., Braak E. (1998). Evolution of neuronal changes in the course of Alzheimer’s disease. J. Neural Transm. Suppl. 53, 127–140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

