Subcutaneous preconditioning increases invasion and metastatic dissemination in mouse colorectal cancer models
- PMID: 24487410
- PMCID: PMC3944498
- DOI: 10.1242/dmm.013995
Subcutaneous preconditioning increases invasion and metastatic dissemination in mouse colorectal cancer models
Abstract
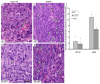
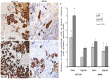
Mouse colorectal cancer (CRC) models generated by orthotopic microinjection of human CRC cell lines reproduce the pattern of lymphatic, haematological and transcoelomic spread but generate low metastatic efficiency. Our aim was to develop a new strategy that could increase the metastatic efficiency of these models. We used subcutaneous implantation of the human CRC cell lines HCT116 or SW48 prior to their orthotopic microinjection in the cecum of nude mice (SC+ORT). This subcutaneous preconditioning significantly enhanced metastatic dissemination. In the HCT116 model it increased the number and size of metastatic foci in lymph nodes, lung, liver and peritoneum, whereas, in the SW48 model, it induced a shift from non-metastatic to metastatic. In both models the number of apoptotic bodies in the primary tumour in the SC+ORT group was significantly reduced compared with that in the direct orthotopic injection (ORT) group. Moreover, in HCT116 tumours the number of keratin-positive tumour buddings and single epithelial cells increased at the invasion front in SC+ORT mice. In the SW48 tumour model, we observed a trend towards a higher number of tumour buds and single cells in the SC+ORT group but this did not reach statistical significance. At a molecular level, the enhanced metastatic efficiency observed in the HCT116 SC+ORT model was associated with an increase in AKT activation, VEGF-A overexpression and downregulation of β1 integrin in primary tumour tissue, whereas, in SW48 SC+ORT mice, the level of expression of these proteins remained unchanged. In summary, subcutaneous preconditioning increased the metastatic dissemination of both orthotopic CRC models by increasing tumour cell survival and invasion at the tumour invasion front. This approach could be useful to simultaneously study the mechanisms of metastases and to evaluate anti-metastatic drugs against CRC.
Keywords: Collective invasion; Colorectal cancer model; Metastasis; Orthotopic injection; Single tumour cell; Subcutaneous preconditioning.
Figures


References
-
- Adell G. C., Zhang H., Evertsson S., Sun X. F., Stål O. H., Nordenskjöld B. A. (2001). Apoptosis in rectal carcinoma: prognosis and recurrence after preoperative radiotherapy. Cancer 91, 1870–1875 - PubMed
-
- Bacac M., Stamenkovic I. (2008). Metastatic cancer cell. Annu. Rev. Pathol. 3, 221–247 - PubMed
-
- Bendardaf R., Ristamäki R., Kujari H., Laine J., Lamlum H., Collan Y., Pyrhönen S. (2003). Apoptotic index and bcl-2 expression as prognostic factors in colorectal carcinoma. Oncology 64, 435–442 - PubMed
-
- Bosch R., Moreno M. J., Dieguez-Gonzalez R., Céspedes M. V., Gallardo A., Nomdedeu J., Pavón M. A., Espinosa I., Mangues M. A., Sierra J., et al. (2012). Subcutaneous passage increases cell aggressiveness in a xenograft model of diffuse large B cell lymphoma. Clin. Exp. Metastasis 29, 339–347 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

