Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains
- PMID: 24487536
- PMCID: PMC3993151
- DOI: 10.1128/AEM.03627-13
Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains
Abstract
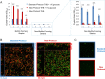
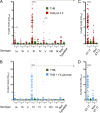
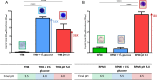
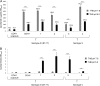
Streptococcus agalactiae, also known as group B Streptococcus (GBS), is a primary colonizer of the anogenital mucosa of up to 40% of healthy women and an important cause of invasive neonatal infections worldwide. Among the 10 known capsular serotypes, GBS type III accounts for 30 to 76% of the cases of neonatal meningitis. In recent years, the ability of GBS to form biofilm attracted attention for its possible role in fitness and virulence. Here, a new in vitro biofilm formation protocol was developed to guarantee more stringent conditions, to better discriminate between strong-, low-, and non-biofilm-forming strains, and to facilitate interpretation of data. This protocol was used to screen the biofilm-forming abilities of 366 GBS clinical isolates from pregnant women and from neonatal infections of different serotypes in relation to medium composition and pH. The results identified a subset of isolates of serotypes III and V that formed strong biofilms under acidic conditions. Importantly, the best biofilm formers belonged to serotype III hypervirulent clone ST-17. Moreover, the abilities of proteinase K to strongly inhibit biofilm formation and to disaggregate mature biofilms suggested that proteins play an essential role in promoting GBS biofilm initiation and contribute to biofilm structural stability.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

