Skilled reaching relies on a V2a propriospinal internal copy circuit
- PMID: 24487617
- PMCID: PMC4230338
- DOI: 10.1038/nature13021
Skilled reaching relies on a V2a propriospinal internal copy circuit
Abstract
The precision of skilled forelimb movement has long been presumed to rely on rapid feedback corrections triggered by internally directed copies of outgoing motor commands, but the functional relevance of inferred internal copy circuits has remained unclear. One class of spinal interneurons implicated in the control of mammalian forelimb movement, cervical propriospinal neurons (PNs), has the potential to convey an internal copy of premotor signals through dual innervation of forelimb-innervating motor neurons and precerebellar neurons of the lateral reticular nucleus. Here we examine whether the PN internal copy pathway functions in the control of goal-directed reaching. In mice, PNs include a genetically accessible subpopulation of cervical V2a interneurons, and their targeted ablation perturbs reaching while leaving intact other elements of forelimb movement. Moreover, optogenetic activation of the PN internal copy branch recruits a rapid cerebellar feedback loop that modulates forelimb motor neuron activity and severely disrupts reaching kinematics. Our findings implicate V2a PNs as the focus of an internal copy pathway assigned to the rapid updating of motor output during reaching behaviour.
Figures
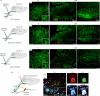
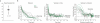
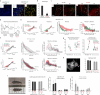
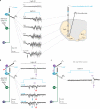
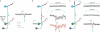

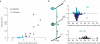

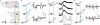

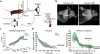


Comment in
-
Motor systems: reaching out and grasping the molecular tools.Curr Biol. 2014 Mar 31;24(7):R269-71. doi: 10.1016/j.cub.2014.02.048. Curr Biol. 2014. PMID: 24698373
References
-
- Iwaniuk AN, Whishaw IQ. On the origin of skilled forelimb movements. Trends Neurosci. 2000;23:372–376. doi:S0166-2236(00)01618-0 [pii] - PubMed
-
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. Journal of experimental psychology. 1954;47:381–391. - PubMed
-
- Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42:223–227. - PubMed
-
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain : a journal of neurology. 1968;91:1–14. - PubMed
-
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain : a journal of neurology. 1968;91:15–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

