Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse
- PMID: 24487619
- PMCID: PMC3949170
- DOI: 10.1038/nature12951
Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse
Abstract
The recognition events that mediate adaptive cellular immunity and regulate antibody responses depend on intercellular contacts between T cells and antigen-presenting cells (APCs). T-cell signalling is initiated at these contacts when surface-expressed T-cell receptors (TCRs) recognize peptide fragments (antigens) of pathogens bound to major histocompatibility complex molecules (pMHC) on APCs. This, along with engagement of adhesion receptors, leads to the formation of a specialized junction between T cells and APCs, known as the immunological synapse, which mediates efficient delivery of effector molecules and intercellular signals across the synaptic cleft. T-cell recognition of pMHC and the adhesion ligand intercellular adhesion molecule-1 (ICAM-1) on supported planar bilayers recapitulates the domain organization of the immunological synapse, which is characterized by central accumulation of TCRs, adjacent to a secretory domain, both surrounded by an adhesive ring. Although accumulation of TCRs at the immunological synapse centre correlates with T-cell function, this domain is itself largely devoid of TCR signalling activity, and is characterized by an unexplained immobilization of TCR-pMHC complexes relative to the highly dynamic immunological synapse periphery. Here we show that centrally accumulated TCRs are located on the surface of extracellular microvesicles that bud at the immunological synapse centre. Tumour susceptibility gene 101 (TSG101) sorts TCRs for inclusion in microvesicles, whereas vacuolar protein sorting 4 (VPS4) mediates scission of microvesicles from the T-cell plasma membrane. The human immunodeficiency virus polyprotein Gag co-opts this process for budding of virus-like particles. B cells bearing cognate pMHC receive TCRs from T cells and initiate intracellular signals in response to isolated synaptic microvesicles. We conclude that the immunological synapse orchestrates TCR sorting and release in extracellular microvesicles. These microvesicles deliver transcellular signals across antigen-dependent synapses by engaging cognate pMHC on APCs.
Conflict of interest statement
Competing financial interests: The authors declare no competing financial interests.
Figures
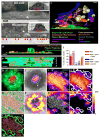
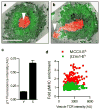
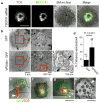
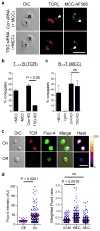
References
-
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. - PubMed
-
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–5. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. - PubMed
-
- Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PN2 EY016586/EY/NEI NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- AI045757/AI/NIAID NIH HHS/United States
- AI088377/AI/NIAID NIH HHS/United States
- R01 AI043542/AI/NIAID NIH HHS/United States
- AI055037/AI/NIAID NIH HHS/United States
- K99 AI093884/AI/NIAID NIH HHS/United States
- R01 AI055037/AI/NIAID NIH HHS/United States
- EY016586/EY/NEI NIH HHS/United States
- R01 AI088377/AI/NIAID NIH HHS/United States
- K99AI093884/AI/NIAID NIH HHS/United States
- R37 AI043542/AI/NIAID NIH HHS/United States
- P01 AI045757/AI/NIAID NIH HHS/United States
- AI043542/AI/NIAID NIH HHS/United States
- 100262/WT_/Wellcome Trust/United Kingdom
- AI093884/AI/NIAID NIH HHS/United States
- R21 AI055037/AI/NIAID NIH HHS/United States
- R00 AI093884/AI/NIAID NIH HHS/United States
- PN1 EY016586/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

