Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials
- PMID: 24487777
- PMCID: PMC4180680
- DOI: 10.1038/ncomms4206
Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials
Abstract
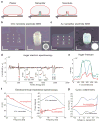
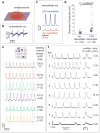
Intracellular recording of action potentials is important to understand electrically-excitable cells. Recently, vertical nanoelectrodes have been developed to achieve highly sensitive, minimally invasive and large-scale intracellular recording. It has been demonstrated that the vertical geometry is crucial for the enhanced signal detection. Here we develop nanoelectrodes of a new geometry, namely nanotubes of iridium oxide. When cardiomyocytes are cultured upon those nanotubes, the cell membrane not only wraps around the vertical tubes but also protrudes deep into the hollow centre. We show that this nanotube geometry enhances cell-electrode coupling and results in larger signals than solid nanoelectrodes. The nanotube electrodes also afford much longer intracellular access and are minimally invasive, making it possible to achieve stable recording up to an hour in a single session and more than 8 days of consecutive daily recording. This study suggests that the nanoelectrode performance can be significantly improved by optimizing the electrode geometry.
Conflict of interest statement
Figures



References
-
- Sakmann B, Neher E. Single-channel recording. 2. Springer; New York, NY: 2009.
-
- Thomas CA, Jr, Springer PA, Loeb GE, Berwald-Netter Y, Okun LM. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp Cell Res. 1972;74:61–66. - PubMed
-
- Pine J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J Neurosci Methods. 1980;2:19–31. - PubMed
-
- Connolly P, Clark P, Curtis AS, Dow JA, Wilkinson CD. An extracellular microelectrode array for monitoring electrogenic cells in culture. Biosens Bioelectron. 1990;5:223–234. - PubMed
-
- Amin AS, Tan HL, Wilde AA. Cardiac ion channels in health and disease. Heart Rhythm. 2010;7:117–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

