Drug resistance of a viral population and its individual intrahost variants during the first 48 hours of therapy
- PMID: 24488144
- PMCID: PMC4215939
- DOI: 10.1038/clpt.2014.20
Drug resistance of a viral population and its individual intrahost variants during the first 48 hours of therapy
Abstract
Using hepatitis C virus (HCV) and interferon (IFN) resistance as a proof of concept, we have devised a new method for calculating the effect of a drug on a viral population, as well as the resistance of the population's individual intrahost variants. By means of next-generation sequencing, HCV variants were obtained from sera collected at nine time points from 16 patients during the first 48 h after injection of IFN-α. IFN-resistance coefficients were calculated for individual variants using changes in their relative frequencies, and for the entire intrahost viral population using changes in viral titer. Population-wide resistance and presence of IFN-resistant variants were highly associated with pegylated IFN-α2a/ribavirin treatment outcome at week 12 (P = 3.78 × 10(-5) and 0.0114, respectively). This new method allows an accurate measurement of resistance based solely on changes in viral titer or the relative frequency of intrahost viral variants during a short observation time.
Conflict of interest statement
Figures

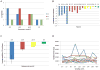

Similar articles
-
Distinct hepatitis C virus kinetics in HIV-infected patients treated with ribavirin plus either pegylated interferon alpha2a or alpha2b.Antivir Ther. 2008;13(4):511-7. Antivir Ther. 2008. PMID: 18672529 Clinical Trial.
-
Virological response in patients with hepatitis C virus genotype 1b and a high viral load: impact of peginterferon-alpha-2a plus ribavirin dose reductions and host-related factors.Clin Drug Investig. 2008;28(1):9-16. doi: 10.2165/00044011-200828010-00002. Clin Drug Investig. 2008. PMID: 18081356
-
Genetic heterogeneity of hepatitis C virus in association with antiviral therapy determined by ultra-deep sequencing.PLoS One. 2011;6(9):e24907. doi: 10.1371/journal.pone.0024907. Epub 2011 Sep 22. PLoS One. 2011. PMID: 21966381 Free PMC article.
-
Pegylated IFN-alpha2a and ribavirin in the treatment of hepatitis C.Expert Rev Anti Infect Ther. 2009 Oct;7(8):925-35. doi: 10.1586/eri.09.70. Expert Rev Anti Infect Ther. 2009. PMID: 19803700 Review.
-
Is there still a role for PEG IFN+RBV therapy in patients with HCV genotype 1?Liver Int. 2014 Feb;34 Suppl 1:11-2. doi: 10.1111/liv.12407. Liver Int. 2014. PMID: 24373072 Review.
Cited by
-
Inference of genetic relatedness between viral quasispecies from sequencing data.BMC Genomics. 2017 Dec 6;18(Suppl 10):918. doi: 10.1186/s12864-017-4274-5. BMC Genomics. 2017. PMID: 29244009 Free PMC article.
-
Analysis of heterogeneous genomic samples using image normalization and machine learning.BMC Genomics. 2020 Dec 21;21(Suppl 6):405. doi: 10.1186/s12864-020-6661-6. BMC Genomics. 2020. PMID: 33349236 Free PMC article.
-
Accurate assembly of minority viral haplotypes from next-generation sequencing through efficient noise reduction.Nucleic Acids Res. 2021 Sep 27;49(17):e102. doi: 10.1093/nar/gkab576. Nucleic Acids Res. 2021. PMID: 34214168 Free PMC article.
-
Using earth mover's distance for viral outbreak investigations.BMC Genomics. 2020 Dec 16;21(Suppl 5):582. doi: 10.1186/s12864-020-06982-4. BMC Genomics. 2020. PMID: 33327932 Free PMC article.
-
Advanced molecular surveillance of hepatitis C virus.Viruses. 2015 Mar 13;7(3):1153-88. doi: 10.3390/v7031153. Viruses. 2015. PMID: 25781918 Free PMC article. Review.
References
-
- Said Z, Abdelwahab K. Viral Replication. InTech; 2013. pp. 127–144. ed. D. 10.5772/53707.
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2012 - PubMed
-
- Hsu CS, et al. Early viral kinetics during treatment of chronic hepatitis C virus infection with pegylated interferon alpha plus ribavirin in Taiwan. Intervirology. 2007;50:310–315. - PubMed
-
- Aghemo A, Rumi MG, Colombo M. Pegylated IFN-alpha2a and ribavirin in the treatment of hepatitis C. Expert review of anti-infective therapy. 2009;7:925–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

