Pharmacological interventions for hypertension in children
- PMID: 24488616
- PMCID: PMC11056235
- DOI: 10.1002/14651858.CD008117.pub2
Pharmacological interventions for hypertension in children
Abstract
Background: Hypertension is a major risk factor for stroke, coronary artery disease and kidney damage in adults. There is a paucity of data on the long-term sequelae of persistent hypertension in children, but it is known that children with hypertension have evidence of end organ damage and are at risk of hypertension into adulthood. The prevalence of hypertension in children is rising, most likely due to a concurrent rise in obesity rates. In children with hypertension, non-pharmacological measures are often recommended as first-line therapy, but a significant proportion of children will eventually require pharmacological treatment to reduce blood pressure, especially those with evidence of end organ damage at presentation or during follow-up. A systematic review of the effects of antihypertensive agents in children has not previously been conducted.
Objectives: To determine the dose-related effects of different classes of antihypertensive medications, as monotherapy compared to placebo; as combination therapy compared to placebo or a single medication; or in comparisons of various doses within the same class, on systolic or diastolic blood pressure (or both) in children with hypertension.
Search methods: We searched the Cochrane Hypertension Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 9), Ovid MEDLINE (1946 to October 2013), Ovid EMBASE (1974 to October 2013) and bibliographic citations.
Selection criteria: The selection criteria were deliberately broad due to there being few clinical trials in children. We included randomised controlled trials (RCTs) of at least two weeks duration comparing antihypertensive agents either as monotherapy or combination therapy with either placebo or another medication, or comparing different doses of the same medication, in children with hypertension. Hypertension was defined as an average (over a minimum of three readings) systolic or diastolic blood pressure (or both) on the 95(th) percentile or above for age, height and gender.
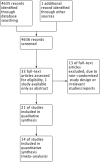
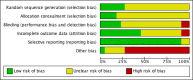
Data collection and analysis: Two authors independently selected relevant studies, extracted data and assessed risk of bias. We summarised data, where possible, using a random-effects model. Formal assessment of heterogeneity was not possible because of insufficient data.
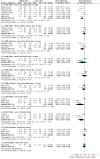
Main results: A total of 21 trials evaluated antihypertensive medications of various drug classes in 3454 hypertensive children with periods of follow-up ranging from three to 24 weeks. There were five RCTs comparing an antihypertensive drug directly with placebo, 12 dose-finding trials, two trials comparing calcium channel blockers with angiotensin receptor blockers, one trial comparing a centrally acting alpha blocker with a diuretic and one trial comparing an angiotensin-converting enzyme inhibitor with an angiotensin receptor blocker. No randomised trial was identified that evaluated the effectiveness of antihypertensive medications on target end organ damage. The trials were of variable quality and most were funded by pharmaceutical companies.Among the angiotensin receptor blockers, candesartan (one trial, n = 240), when compared to placebo, reduced systolic blood pressure by 6.50 mmHg (95% confidence interval (CI) -9.44 to -3.56) and diastolic blood pressure by 5.50 mmHg (95% CI -9.62 to -1.38) (low-quality evidence). High dose telmisartan (one trial, n = 76), when compared to placebo, reduced systolic blood pressure by -8.50 (95% CI -13.79 to -3.21) but not diastolic blood pressure (-4.80, 95% CI -9.50 to 0.10) (low-quality evidence). Beta blocker (metoprolol, one trial, n = 140), when compared with placebo , significantly reduced systolic blood pressure by 4.20 mmHg (95% CI -8.12 to -0.28) but not diastolic blood pressure (-3.20 mmHg 95% CI -7.12 to 0.72) (low-quality evidence). Beta blocker/diuretic combination (Bisoprolol/hydrochlorothiazide, one trial, n = 94)when compared with placebo , did not result in a significant reduction in systolic blood pressure (-4.0 mmHg, 95% CI -8.99 to -0.19) but did have an effect on diastolic blood pressure (-4.50 mmHg, 95% CI -8.26 to -0.74) (low-quality evidence). Calcium channel blocker (extended-release felodipine,one trial, n = 133) was not effective in reducing systolic blood pressure (-0.62 mmHg, 95% CI -2.97 to 1.73) or diastolic blood pressure (-1.86 mmHg, 95% CI -5.23 to 1.51) when compared with placebo. Further, there was no consistent dose response observed among any of the drug classes. The adverse events associated with the antihypertensive agents were mostly minor and included headaches, dizziness and upper respiratory infections.
Authors' conclusions: Overall, there are sparse data informing the use of antihypertensive agents in children, with outcomes reported limited to blood pressure and not end organ damage. The most data are available for candesartan, for which there is low-quality evidence of a modest lowering effect on blood pressure. We did not find evidence of a consistent dose response relationship for escalating doses of angiotensin receptor blockers, calcium channel blockers or angiotensin-converting enzyme inhibitors. All agents appear safe, at least in the short term.
Conflict of interest statement
None known.
Figures







Update of
References
References to studies included in this review
Batisky 2007 {published data only (unpublished sought but not used)}
-
- Batisky DL, Sorof JM, Sugg J, Llewellyn M, Klibaner M, Hainer JW, et al. Efficacy and safety of extended release metoprolol succinate in hypertensive children 6 to 16 years of age: a clinical trial experience. Journal of Pediatrics 2007;150(2):134‐9. - PubMed
BMS 2005 {unpublished data only}
-
- Bristol‐Meyers Squibb. United States Food and Drug Administration. Summaries of Medical and Clinical Pharmacology Reviews. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources... 2005.
Falkner 1983 {published data only}
-
- Falkner B, Onesti G, Lowenthal DT, Affrime MB. Use of clonidine monotherapy (versus diuretics) in adolescent hypertension. Chest 1983;83:425‐7. - PubMed
Flynn 2004 {published data only (unpublished sought but not used)}
-
- Flynn JT, Newburger JW, Daniels SR, Sanders SP, Portman RJ, Hogg RJ, et al. A randomized, placebo‐controlled trial of amlodipine in children with hypertension. Journal of Pediatrics 2004;145(3):353‐9. - PubMed
Flynn 2008 {published data only (unpublished sought but not used)}
-
- Flynn JT, Meyers KE, Neto JP, Paula Meneses R, Zurowska A, Bagga A, et al. Efficacy and safety of the angiotensin receptor blocker valsartan in children with hypertension aged 1 to 5 years. Hypertension 2008;52(2):222‐8. - PubMed
Gartenmann 2003 {published data only (unpublished sought but not used)}
-
- Gartenmann AC, Fossali E, Vigier RO, Simonetti GD, Schmidtko J, Edefonti A, et al. Better renoprotective effect of angiotensin II antagonist compared to dihydropyridine calcium channel blocker in childhood. Kidney International 2003;64(4):1450‐4. - PubMed
Hazan 2010a {published and unpublished data}
-
- Hazan L. Personal correspondence October 2010.
-
- Hazan L, Hernandez Rodriguez OA, Bhorat AE, Miyazaki K, Tao B, Heyrman RA, Assessment of Efficacy and Safety of Olmesartan in Pediatric Hypertension (AESOP) Study Group. A double‐blind, dose‐response study of the efficacy and safety of olmesartan medoxomil in children and adolescents with hypertension. Hypertension 2010;55(6):1323‐30. - PubMed
Hazan 2010b {published and unpublished data}
-
- Hazan L, Hernandez Rodriguez OA, Bhorat AE, Miyazaki K, Tao B, Heyrman R, Assessment of Efficacy and Safety of Olmesartan in Pediatric Hypertension (AESOP) Study Group. A double‐blind, dose‐response study of the efficacy and safety of olmesartan medoxomil in children and adolescents with hypertension. Hypertension 2010;55(6):1323‐30. - PubMed
Li 2004 {published and unpublished data}
-
- Li JS. Personal communication 11 April 2010.
-
- Li JS, Berezny K, Kilaru R, Hazan L, Portman R, Hogg R, et al. Is the extrapolated adult dose of fosinopril safe and effective in treating hypertensive children?. Hypertension 2004;44(3):289‐93. - PubMed
Li 2010 {published data only (unpublished sought but not used)}
-
- Li JS, Flynn JT, Portman R, Davis I, Ogawa M, Shi H, Pressler ML. The efficacy and safety of the novel aldosterone antagonist eplerenone in children with hypertension: a randomized, double‐blind, dose‐response study. Journal of Pediatrics. United States: Division of Pediatric Cardiology and Duke Clinical Research Institute, Duke University Medical Center, Durham, NC 27710, USA. jennifer.li@duke.edu, 2010; Vol. 157, issue 2:282‐7. - PubMed
Schaefer 2010 {published data only}
-
- Schaefer F, Walle JVD, Zurowska A, Gimpel C, Hoeck KV, Drozdz D, et al for the Candesartan in Children with Hypertension (CINCH) Investigators. Efficacy, safety, and pharmacokinetics of candesartan cilexetil in hypertensive children from 1 to less than 6 years of age. Journal of Hypertension 2010;28(5):1083‐90. - PubMed
Schaefer 2011 {published data only (unpublished sought but not used)}
-
- Schaefer F, Litwin M, Zachwieja J, Zurowska A, Turi S, Grosso A, et al. Efficacy and safety of valsartan compared to enalapril in hypertensive children: a 12‐week, randomized, double‐blind, parallel‐group study. Journal of Hypertension 2011;29:2484‐90. - PubMed
Shahinfar 2005 {published data only (unpublished sought but not used)}
-
- Shahinfar S, Cano F, Soffer BA, Ahmed T, Santoro EP, Zhang Z, et al. A double‐blind, dose‐response study of losartan in hypertensive children. American Journal of Hypertension 2005;18(2 Pt 1):183‐90. - PubMed
Soffer 2003 {published data only (unpublished sought but not used)}
-
- Soffer B, Zhang Z, Miller K, Vogt BA, Shahinfar S. A double‐blind, placebo‐controlled, dose‐response study of the effectiveness and safety of lisinopril for children with hypertension. American Journal of Hypertension 2003;16(10):795‐800. - PubMed
Sorof 2002 {published data only (unpublished sought but not used)}
-
- Sorof JM, Cargo P, Graepel J, Humphrey D, King E, Rolf C, et al. Beta‐blocker/thiazide combination for treatment of hypertensive children: a randomized double‐blind, placebo‐controlled trial. Pediatric Nephrology 2002;17(5):345‐50. - PubMed
Trachtman 2003 {published data only (unpublished sought but not used)}
-
- Trachtman H, Frank R, Mahan JD, Portman R, Restaino I, Matoo TK, et al. Clinical trial of extended‐release felodipine in pediatric essential hypertension. Pediatric Nephrology 2003;18(6):548‐53. - PubMed
Trachtman 2008 {published and unpublished data}
-
- Trachtman H. Personal communication April 2010.
-
- Trachtman H, Hainer JW, Sugg J, Teng R, Sorof JM, Radcliffe J for the Candesartan in Children with Hypertension (CINCH) Investigators. Efficacy, safety, and pharmacokinetics of candesartan cilexetil in hypertensive children aged 6 to 17 years. Journal of Clinical Hypertension 2008;10(10):743‐50. - PMC - PubMed
Webb 2010 {published data only (unpublished sought but not used)}
Webb 2013 {published data only (unpublished sought but not used)}
Wells 2002 {published and unpublished data}
-
- Wells T, Frame V, Soffer B, Shaw W, Zhang Z, Herrera P, et al. A double‐blind, placebo‐controlled, dose‐response study of the effectiveness and safety of enalapril for children with hypertension. Journal of Clinical Pharmacology 2002;42(8):870‐80. - PubMed
Wells 2010 {published data only}
-
- Wells TG, Portman R, Norman P, Haertter S, Davidai G, Fei Wang. Safety, efficacy, and pharmacokinetics of telmisartan in pediatric patients with hypertension. Clinical Pediatrics 2010;49(10):938‐46. [PUBMED: 20724342] - PubMed
References to studies excluded from this review
Bachmann 1984 {published data only}
-
- Bachmann H. Propranolol versus chlorthalidone‐‐a prospective therapeutic trial in children with chronic hypertension. Helvetica Paediatrica Acta 1984;39(1):55‐61. - PubMed
Berenson 1990 {published data only}
-
- Berenson GS, Shear CL, Chiang YK, Webber LS, Voors AW. Combined low‐dose medication and primary intervention over a 30‐month period for sustained high blood pressure in childhood. American Journal of the Medical Sciences 1990;299(2):79‐86. - PubMed
Chan 2012 {published data only}
-
- Chan EK, Quach J, Mensah FK, Sung V, Cheung M, Wake M. Dark chocolate for children’s blood pressure: randomised trial. Archives of Disease in Childhood 2012;97:637‐40. - PubMed
Cichocka 1993 {published data only}
-
- Cichocka E, Januszewicz P, Wyszynska T. Evaluation of the efficacy and tolerance of three antihypertensive agents used as single‐drug therapy, nifedipine, prazosin and acebutolol in severe, idiopathic hypertension in adolescents. Annales de Pediatrie 1993;40(2):119‐26. - PubMed
Ellis 2004 {published data only}
-
- Ellis D, Moritz ML, Vats A, Janosky JE. Antihypertensive and renoprotective efficacy and safety of losartan. A long‐term study in children with renal disorders. American Journal of Hypertension 2004;17(10):928‐35. - PubMed
Feig 2008 {published data only}
Friedman 1980 {published data only}
-
- Friedman A, Chesney RW, Ball D, Goodfriend T. Effective use of captopril (angiotensin I‐converting enzyme inhibitor) in severe childhood hypertension. Journal of Pediatrics 1980;97(4):664‐7. - PubMed
Meyers 2011 {published data only}
Moncica 1995 {published data only}
Morsi 1992 {published data only}
-
- Morsi MR, Madina EH, Anglo AA, Soliman AT. Evaluation of captopril versus reserpine and frusemide in treating hypertensive children with acute post‐streptococcal glomerulonephritis. Acta Paediatrica 1992;81(2):145‐9. - PubMed
Rogan 2000 {published data only}
-
- Rogan JW, Lyszkiewicz DA, Blowey D, Khattak S, Arbus GS, Koren G. A randomized prospective crossover trial of amlodipine in pediatric hypertension. Pediatric Nephrology 2000;14(12):1083‐7. - PubMed
Sinaiko 1977 {published data only}
-
- Sinaiko AR, Mirkin BL. Management of severe childhood hypertension with minoxidil: a controlled clinical study. Journal of Pediatrics 1977;91(1):138‐42. - PubMed
Sinaiko 1983 {published data only}
-
- Sinaiko AR, Mirkin BL, Hendrick DA, Green TP, O'Dea RF. Antihypertensive effect and elimination kinetics of captopril in hypertensive children with renal disease. Journal of Pediatrics 1983;103(5):799‐805. - PubMed
White 2003 {published data only}
-
- White CT, Macpherson CF, Hurley RM, Matsell DG. Antiproteinuric effects of enalapril and losartan: a pilot study. Pediatric Nephrology 2003;18(10):1038‐43. - PubMed
Wühl 2009 {published data only}
-
- ESCAPE Trial Group, Wühl E, Trivelli A, Picca S, Litwin M, Peco‐Antic A, Zurowska A, et al. Strict blood‐pressure control and progression of renal failure in children. New England Journal of Medicine 2009;361(17):1639‐50. - PubMed
Additional references
ALLHAT 2002
-
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐97. - PubMed
Assadi 2007
-
- Assadi F. Effect of microalbuminuria lowering on regression of left ventricular hypertrophy in children and adolescents with essential hypertension. Pediatric Cardiology 2007;28(1):27‐33. - PubMed
Benjamin 2008
Bovet 2006
-
- Bovet P, Chiolero A, Madeleine G, Gabriel A, Stettler N. Marked increase in the prevalence of obesity in children of the Seychelles, a rapidly developing country, between 1998 and 2004. International Journal of Pediatric Obesity 2006;1(2):120‐8. - PubMed
Chobanian 2003
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560‐72. - PubMed
Couch 2008
-
- Couch SC, Saelens BE, Levin L, Dart K, Falciglia G, Daniels SR. The efficacy of a clinic‐based behavioral nutrition intervention emphasizing a DASH‐type diet for adolescents with elevated blood pressure. Journal of Pediatrics 2008;152(4):494‐501. - PubMed
Daniels 1991
-
- Daniels SR, Lipman MJ, Burke MJ, Loggie JM. The prevalence of retinal vascular abnormalities in children and adolescents with essential hypertension. American Journal of Ophthalmology 1991;111(2):205‐8. - PubMed
Denzer 2004
-
- Denzer C, Reithofer E, Wabitsch M, Widhalm K. The outcome of childhood obesity management depends highly upon patient compliance. European Journal of Pediatrics 2004;163(2):99‐104. - PubMed
Din‐Dzietham 2007
-
- Din‐Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007;116(13):1488‐96. - PubMed
Flynn 2011
-
- Flynn J. Management of hypertension in the young: role of antihypertensive medications. Journal of Cardiovascular Pharmacology 2011;58(2):111‐20. - PubMed
Hansson 1999
-
- Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999;354:1751‐6. - PubMed
Hansson 2000
-
- Hansson L, Hedner T, Lund‐Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359‐65. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laird 1981
-
- Laird WP, Fixler DE. Left ventricular hypertrophy in adolescents with elevated blood pressure: assessment by chest roentgenography, electrocardiography, and echocardiography. Pediatrics 1981;67(2):255‐9. - PubMed
Lande 2006
-
- Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension 2006;48(1):40‐4. - PubMed
Liberman 2009
-
- Liberman JN, Berger JE, Lewis M. Prevalence of antihypertensive, antidiabetic, and dyslipidemic prescription medication use among children and adolescents. Archives of Pediatrics and Adolescent Medicine 2009;163:357‐64. - PubMed
Lipszyc 2011
-
- Lipszyc D, Parekh RS. Hypertension in children and adolescents – diagnostic challenges and management. US Nephrol 2011;6(2):110‐5.
McNiece 2007
National High Blood Pressure Group 2004
-
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555‐76. - PubMed
Ogden 2006
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999‐2004. JAMA 2006;295(13):1549‐55. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sorof 2003
-
- Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal‐medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics 2003;111(1):61‐6. - PubMed
Taddei 2011
-
- Taddei S, Bruno RM, Ghiadoni L. The correct administration of antihypertensive drugs according to the principles of clinical pharmacology. American Journal of Cardiovascular Drugs 2011;11:13‐20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

